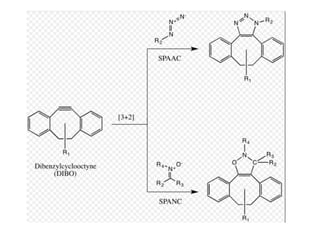
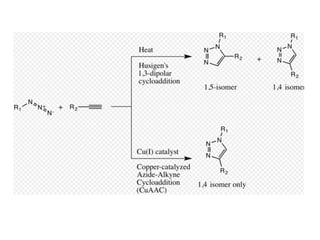
Click chemistry, introduced by K.B. Sharpless in 2001, is a chemical approach that emphasizes rapid and reliable creation of substances through modular reactions with high yields and benign by-products. Key reactions in this field include the copper(I)-catalyzed azide-alkyne cycloaddition and strain-promoted azide-alkyne cycloaddition, which are applied in various areas such as drug discovery, material science, and modification of natural products. The methodology is characterized by simplicity, insensitivity to reaction conditions, and high atom economy.






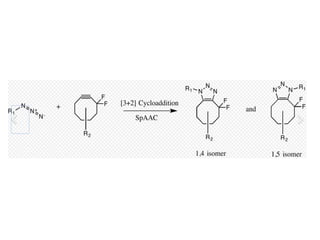
![Strain-promoted azide-alkyne
cycloaddition (SPAAC)
• Instead of using Cu(I) to activate the alkyne,
the alkyne is introduced in a strained
difluorooctyne (DIFO), having electron-with
• This reaction proceeds as a concerted [3+2]
cycloaddition drawing fluorines destabilize
the alkyne.
• The reaction rate is slower than CuAAC.
Moreover, because the synthesis of
cyclooctynes often gives low yield.](https://image.slidesharecdn.com/clickchemistry-200609080122/85/CLICK-CHEMISTRY-9-320.jpg)