Thermodynamics Lecture on General Relations and the Joule-Thomson Coefficient
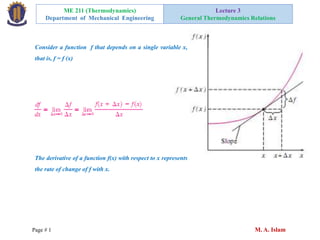
- 1. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 1 M. A. Islam Consider a function f that depends on a single variable x, that is, f = f (x) The derivative of a function f(x) with respect to x represents the rate of change of f with x.
- 2. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 2 M. A. Islam Example: The Cp of ideal gases depends on temperature only, and it is expressed as cp(T ) dh(T )/dT. Determine the cp of air at 300 K, using the enthalpy data from Table A–17 [3]
- 3. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 3 M. A. Islam Partial derivative The variation of z(x, y) with x when y is held constant is called the partial derivative of z with respect to x, and it is expressed as 1) The total differential change (d) of a function and reflects the influence of all variables 2) The partial differential change (𝜕) due to the variation of a single variable Geometric representation of partial derivative Examines the dependence of z on only one of the variables
- 4. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 4 M. A. Islam The fundamental relation for total derivative with partial derivatives Consider a small portion of the surface z(x, y) and for total differential change in z(x, y) for simultaneous changes in x and y. When the independent variables x and y change by ∆ x and ∆ y, respectively, the dependent variable z changes by ∆ z Geometric representation dz for a function z(x, y) 𝒅𝒛 = 𝝏𝒛 𝝏𝒙 𝒚 𝒅𝒙 + 𝝏𝒛 𝝏𝒚 𝒙 𝒅𝒚
- 5. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 5 M. A. Islam Example: Total Differential versus Partial Differential: Consider air at 300 K and 0.86 m3/kg. The state of air changes to 302 K and 0.87 m3/kg as a result of some disturbance. Using Eq., estimate the change in the pressure of air
- 6. Page # 6 M. A. Islam ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General thermodynamics relation
- 7. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 7 M. A. Islam
- 8. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 8 M. A. Islam
- 9. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 9 M. A. Islam Example: Using the ideal-gas equation of state, verify (a) the cyclic relation and (b) the reciprocity relation at constant P.
- 10. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 10 M. A. Islam Maxwell Relations The equations that relate the partial derivatives of properties P, v, T, and s of a simple compressible system to each other are called the Maxwell relations. They are obtained from the four Gibbs equations by exploiting the exactness of the differentials of thermodynamic properties. hey are extremely valuable in thermodynamics because they provide a means of determining the change in entropy,which cannot be measured directly,by simply measuring the changes in properties P, v,and T. Note that the Maxwell relations given above are limited to simple compressible systems. However, other similar relations can be written just as easily for nonsimple systems such as those involving electrical, magnetic, and other effects
- 11. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 11 M. A. Islam Maxwell relations 𝒅𝑼 = 𝑻𝒅𝑺 − 𝑷𝒅𝑽 − 𝜕𝑃 𝜕𝑆 𝑉 = 𝜕𝑇 𝜕𝑉 𝑆 𝒅𝑯 = 𝑻𝒅𝑺 + 𝑽𝒅𝑷 𝜕𝑇 𝜕𝑃 𝑆 = 𝜕𝑉 𝜕𝑆 𝑃 𝑑𝐹 = − 𝑆𝑑𝑇 − 𝑃𝑑𝑉 − 𝜕𝑆 𝜕𝑉 𝑇 = − 𝜕𝑃 𝜕𝑇 𝑃 𝒅𝑮 = −𝑺𝒅𝑻 + 𝑽𝒅𝑷 − 𝜕𝑆 𝜕𝑃 𝑇 = 𝜕𝑉 𝜕𝑇 𝑃 Coefficient Relations Consider a function, Z = Z (X, Y), then dZ = M dX + N dY The coefficients in terms of explicit partial derivatives 𝑴 = 𝝏𝒁 𝝏𝑿 𝒀 and 𝑵 = 𝝏𝒁 𝝏𝒀 𝑿 So, 𝝏𝑴 𝝏𝒀 𝑿 = 𝝏𝑵 𝝏𝑿 𝒀
- 12. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 12 M. A. Islam For Isobaric process: 𝒅𝑯𝑷 = 𝑻𝒅𝑺𝑷 = 𝜹 𝑸𝒓𝒆𝒗, 𝑷 𝒅𝑯 = 𝑻𝒅𝑺 + 𝑽𝒅𝑷 𝒅𝑯 = (𝑻𝒅𝑺 − 𝑷𝒅𝑽) + 𝑷𝒅𝑽 + 𝑽𝒅𝑷 𝒅𝑯 = 𝒅𝑼 + 𝑷𝒅𝑽 + 𝑽𝒅𝑷 H = U +PV For Isothermal process: 𝒅𝑭𝑻 = −𝑷 𝒅𝑽𝑻 = δ𝑾𝑻,𝑻𝒐𝒕𝒂𝒍 𝒅𝑭 = − 𝑺𝒅𝑻 − 𝑷𝒅𝑽 𝒅𝑭 = 𝑻𝒅𝑺 − 𝑷𝒅𝑽 − 𝑻𝒅𝑺 − 𝑺𝒅𝑻 𝒅𝑭 = 𝒅𝑼 − 𝑻𝒅𝑺 − 𝑺𝒅𝑻 F = U -TS The Helmholtz Free energy The Enthalpy
- 13. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 13 M. A. Islam Phase transformation and chemical reaction: 𝛿𝐺𝑇,𝑃 = 𝛿𝑊′ 𝑇, 𝑑𝐺 = −𝑆𝑑𝑇 + 𝑉𝑑𝑃 𝑑𝐺 = 𝑇𝑑𝑆 − 𝑃𝑑𝑉 − 𝑃𝑑𝑉 + 𝑉𝑑𝑃 − 𝑇𝑑𝑆 − 𝑇𝑑𝑆 − 𝑆𝑑𝑇 𝑑𝐺 = 𝑑𝑈 + 𝑃𝑑𝑉 + 𝑉𝑑𝑃 − 𝑇𝑑𝑆 − 𝑆𝑑𝑇 G = H -TS = U + PV - TS
- 14. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 14 M. A. Islam Example: Verify the validity of the last Maxwell relation for steam at 250°C and 300 kPa.
- 15. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 15 M. A. Islam General relations for du, dh, ds, Cv and Cp We choose the internal energy to be a function of T and v; that is, u = u(T, v) and take its total differential
- 16. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 16 M. A. Islam
- 17. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 17 M. A. Islam
- 18. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 18 M. A. Islam
- 19. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 19 M. A. Islam
- 20. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 20 M. A. Islam
- 21. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 21 M. A. Islam
- 22. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 22 M. A. Islam
- 23. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 23 M. A. Islam
- 24. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 24 M. A. Islam
- 25. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 25 M. A. Islam The Joule-Thomson Coefficient When a fluid passes through a restriction such as a porous plug, a capillary tube, or an ordinary valve, its pressure decrease the enthalpy of the fluid remains approximately constant during such a throttling process The fluid may experience a large drop in its temperature as a result of throttling, which forms the basis of operation for refrigerators and air conditioners. The temperature of the fluid may remain unchanged, or it may even increase during a throttling process
- 26. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 26 M. A. Islam The Joule-Thomson Coefficient The temperature behavior of a fluid during a throttling (h = constant)process is described by the Joule-Thomson coefficient, defined as 𝝁 = 𝝏𝑻 𝝏𝑷 𝒉 The Joule-Thomson coefficient is a measure of the change in temperature with pressure during a constant-enthalpy process. Consequences during Throttling process 𝝁 < 𝑻𝒆𝒎𝒑𝒆𝒓𝒂𝒕𝒖𝒓𝒆 𝑰𝒏𝒄𝒓𝒆𝒂𝒔𝒆𝒔 = 𝟎 𝑻𝒆𝒎𝒑𝒆𝒓𝒂𝒕𝒖𝒓𝒆 𝒄𝒐𝒏𝒔𝒕𝒂𝒏𝒕 > 𝑻𝒆𝒎𝒑𝒆𝒓𝒂𝒕𝒖𝒓𝒆 𝒅𝒆𝒄𝒓𝒆𝒂𝒔𝒆𝒔
- 27. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 27 M. A. Islam The Joule-Thomson Coefficient The Joule-Thomson coefficient represents the slope of h constant lines on a T-P diagram Construction of TP Diagram Measure temperature and pressure during throttling processes. Repeat the experiment is for different sizes of porous plugs, each giving a different set of T2 and P2. Plot the temperatures against the pressures gives us an h constant line on a T-P diagramas shown in Figure.
- 28. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 28 M. A. Islam The Joule-Thomson Coefficient Repeating the experiment for different sets of inlet pressure and temperature and plotting the results, a T-P diagram can be constructed for a substance with several h constant lines as in Figure. The inversion line: Some constant-enthalpy lines on the T-P diagram pass through a point of zero slope or zero Joule- Thomson coefficient. Inversion temperature: The temperature at a point where a constant-enthalpy line intersects the inversion line. The maximum inversion temperature: The temperature at the intersection of the P 0 ine (ordinate) and the upper part of the inversion line The slopes of the h constant lines are negative (𝜇 < 0 ) at states to the right of the inversion line and positive (𝝁 > 𝟎 ) to the left of the inversion line
- 29. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 29 M. A. Islam The Joule-Thomson Coefficient Consequences during Throttling The temperature of a fluid increases on the right-hand side of the inversion line (𝑻 ↑, 𝑷 ↓, 𝑫 → , 𝝁 < 𝟎). (process proceeds along a constant-enthalpy line in the direction of decreasing pressure, that is, from right to left.) The fluid temperature decreases on the left-hand side of the inversion line (𝑻 ↓, 𝑷 ↑, 𝑫 ← , 𝝁 > 𝟎) .(Process proceeds along a constant-enthalpy line in the direction of increasing pressure, that is, from left to write) Figure: Constant-enthalpy lines of a substance on a T-P diagram
- 30. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 30 M. A. Islam The Joule-Thomson Coefficient A cooling effect cannot be achieved by throttling unless the fluid is below its maximum inversion temperature. A problem for substances whose maximum inversion temperature is well below room temperature. Example: For hydrogen, the maximum inversion temperature is - 68°C. Thus hydrogen must be cooled below this temperature if any further cooling is to be achieved by throttling. A general relation for the Joule-Thomson coefficient We know, 𝒅𝒉 = 𝑪𝒑𝒅𝑻 + 𝒗 − 𝑻 𝒅𝒗 𝒅𝑷 𝑷 𝒅𝑷 When h = constant, ⇒ 𝝁𝑱𝑻= 𝒅𝑻 𝒅𝑷 𝒉 = − 𝟏 𝑪𝒑 𝒗 − 𝑻 𝒅𝒗 𝒅𝑷 𝑷 𝒅𝑷 Figure: Constant-enthalpy lines of a substance on a T-P diagram
- 31. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 31 M. A. Islam Problem: Show that the Joule-Thomson coefficient of an ideal gas is zero.
- 32. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 32 M. A. Islam Calculating thermodynamic properties such as enthalpy or entropy in terms of other properties that can be measured, the calculations fall into two broad categories: 1) differences in properties between two different phases 2) Changes within a single homogeneous phase
- 33. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 33 M. A. Islam Consider a Carnot-cycle heat engine operating across a small temperature difference between reservoirs at T and T − ∆ T. The corresponding saturation pressures are P and P − ∆P. The Carnot cycle operates with four steady-state devices. In the high-temperature heat-transfer process, the working fluid changes from saturated liquid at 1 to saturated vapor at 2, as shown in the two diagrams of figure. For reversible heat transfer process 𝑞𝐻 = 𝑇𝑆𝑓𝑔 ; 𝑞𝐿 = (𝑇 − ∆𝑇)𝑆𝑓𝑔 ; 𝑊𝑛𝑒𝑡 = 𝑞𝐻 − 𝑞𝐿 = ∆𝑇𝑆𝑓𝑔 As in figure b, each process is steady and reversible 𝑤 = −𝑣 𝑑𝑃
- 34. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 34 M. A. Islam Over all, for the four processes in the cycle 𝑊𝑛𝑒𝑡 = 0 + 2 3 𝑣 𝑑𝑃 + 0 + 4 1 𝑣 𝑑𝑃 = − 𝑣2 + 𝑣3 2 𝑃 − ∆𝑃 − 𝑃 − 𝑣1 + 𝑣4 2 𝑃 − ∆𝑃 − 𝑃 = ∆𝑃 𝑣2+𝑣3 2 − 𝑣1+𝑣4 2 = 𝑞𝐻 − 𝑞𝐿 = ∆𝑇𝑆𝑓𝑔 After simplification and rearranging, ∆𝑃 ∆𝑇 ≈ 𝑆𝑓𝑔 𝑣2+𝑣3 2 − 𝑣1+𝑣4 2 In this limit, ∆𝑇 → 0: 𝑣3 → 𝑣2 = 𝑣𝑔; 𝑣4 → 𝑣1 = 𝑣𝑓 lim ∆𝑇→0 ∆𝑃 ∆𝑇 = 𝑑𝑃𝑠𝑎𝑡 𝑑𝑇 = 𝑆𝑓𝑔 𝑣𝑓𝑔 The heat addition process 1 – 2 is at constant pressure as well as constant temperature 𝒒𝑯 = 𝒉𝒇𝒈 = ∆𝑻𝑺𝒇𝒈 𝒅𝑷𝒔𝒂𝒕 𝒅𝑻 = 𝑺𝒇𝒈 𝒗𝒇𝒈 = 𝒉𝒇𝒈 𝑻𝒗𝒇𝒈 ( Clapeyron equation) This equation establishes the means to cross from one phase to another in 1st or 2nd law calculations
- 35. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 35 M. A. Islam Clapeyron equation for fusion 𝒅𝑷𝒇𝒖𝒔 𝒅𝑻 = 𝑺𝒊𝒇 𝒗𝒊𝒇 = 𝒉𝒊𝒇 𝑻𝒗𝒊𝒇 Clapeyron equation for Sublimation 𝒅𝑷𝑺𝒖𝒃 𝒅𝑻 = 𝑺𝒊𝒈 𝒗𝒊𝒈 = 𝒉𝒊𝒈 𝑻𝒗𝒊𝒈
- 36. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 36 M. A. Islam Determine the sublimation pressure of water vapor at −60◦C using data available in the steam tables.
- 37. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 37 M. A. Islam Exercises 1. Prove the cyclic relation 𝜕𝑧 𝜕𝑥 𝑦 𝜕𝑥 𝜕𝑦 𝑧 𝜕𝑦 𝜕𝑧 𝑥 = - 1
- 38. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 38 M. A. Islam
- 39. ME 211 (Thermodynamics) Department of Mechanical Engineering Lecture 3 General Thermodynamics Relations Page # 39 M. A. Islam