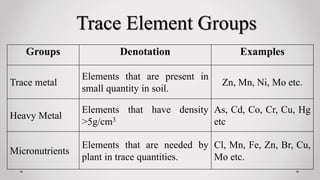
This presentation discusses the role of trace elements in plants. It defines trace elements as chemical elements found in low concentrations in soil, typically less than 100mg/kg. Various trace elements are grouped as trace metals, heavy metals, and micronutrients. Specific trace elements discussed include zinc, copper, chlorine, molybdenum, cobalt, selenium, iodine, boron, iron, lead, fluorine, arsenic, cadmium, nickel, chromium, and manganese. For each element, sources in soil, chemical forms, plant uptake, functions in plants, deficiency and toxicity symptoms are summarized.