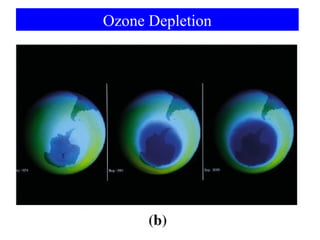
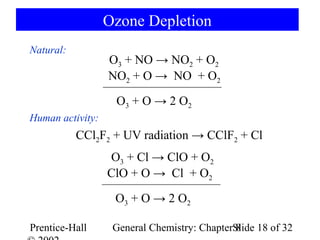
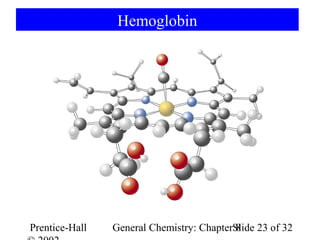
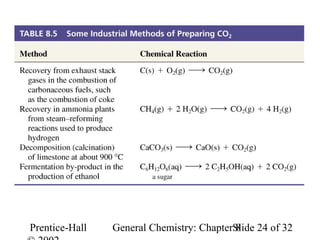
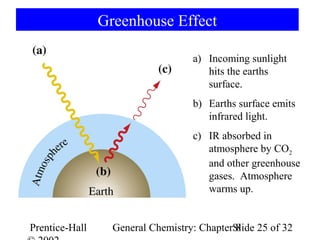
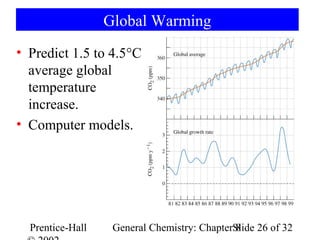
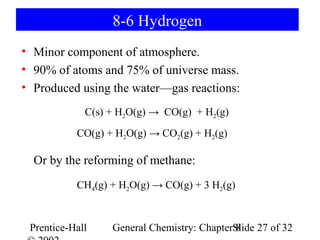
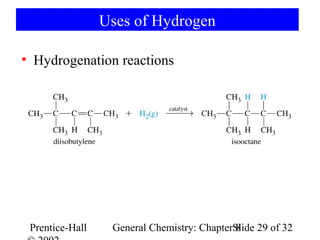
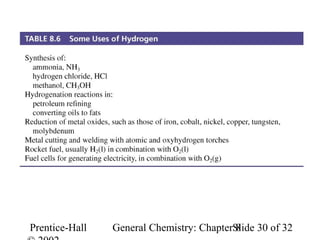
This document is a PowerPoint presentation on Chapter 8 from the textbook "General Chemistry: Principles and Modern Applications" by Petrucci, Harwood, and Herring. The chapter discusses the composition and importance of the atmospheric gases nitrogen, oxygen, noble gases, and hydrogen. It also covers the carbon and nitrogen cycles, as well as chemical processes involving these elements like the Haber-Bosch process and ozone depletion. The presentation concludes with sample questions related to the chapter material.