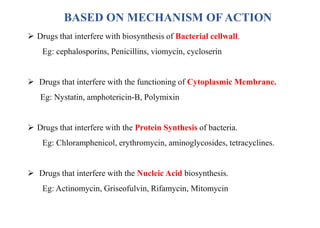
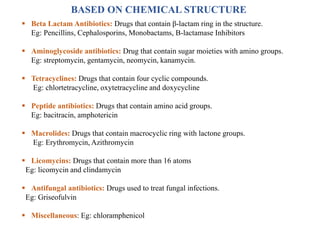
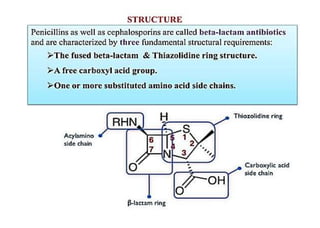
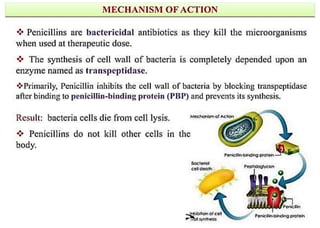
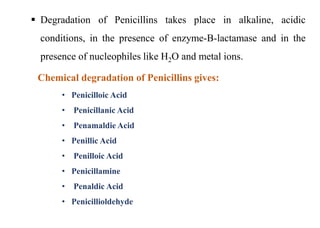
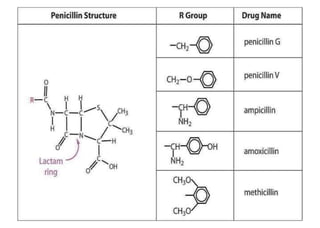
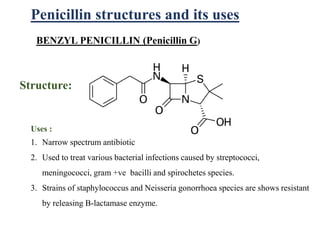
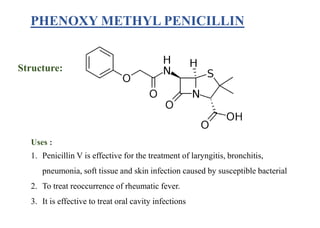
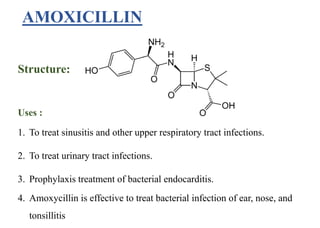
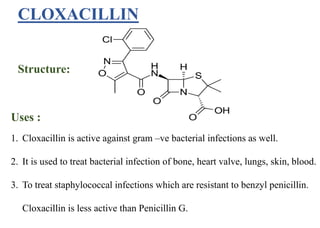
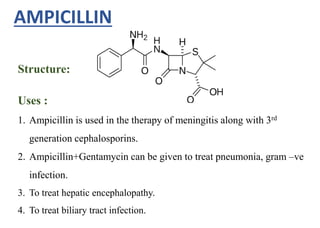
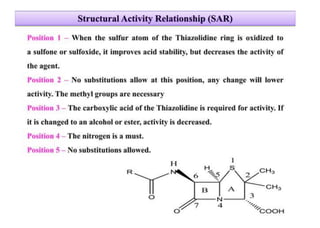
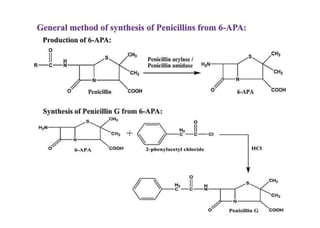
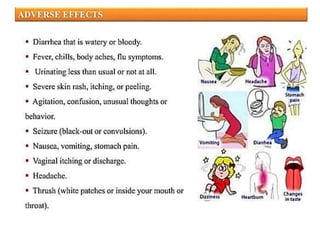
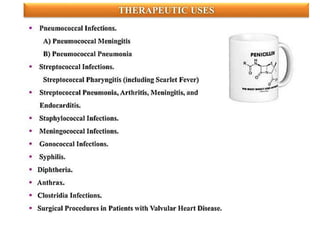
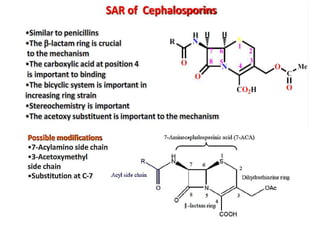
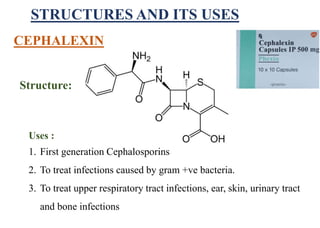
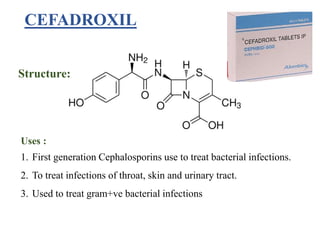
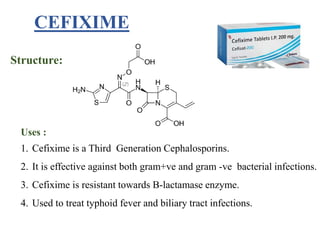
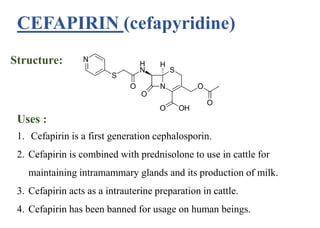
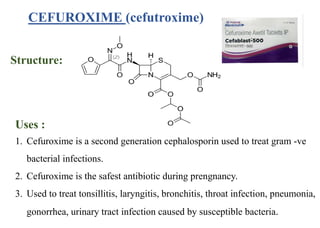
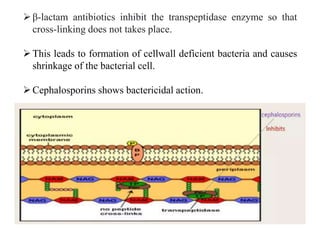
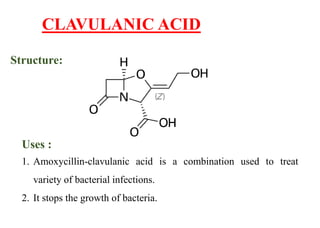
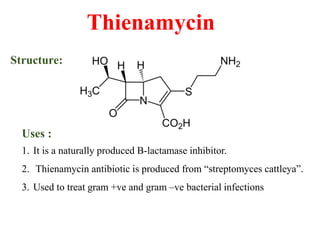
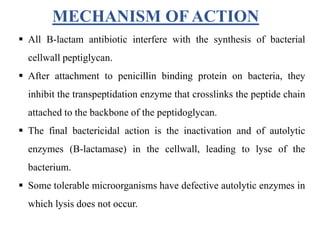
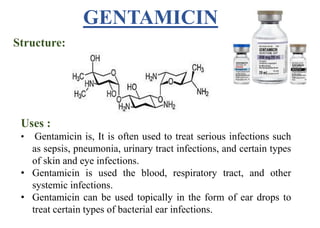
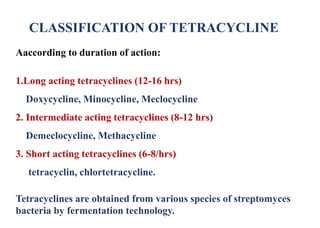
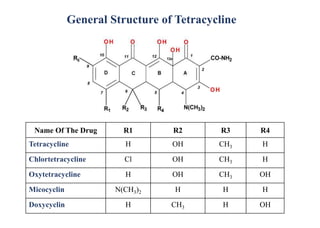
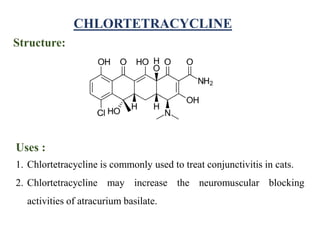
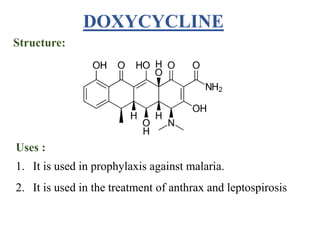
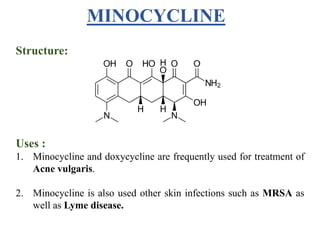
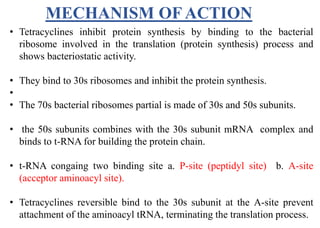
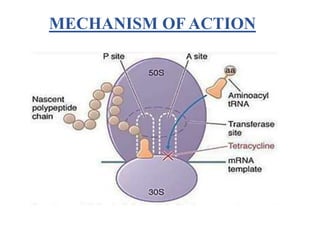
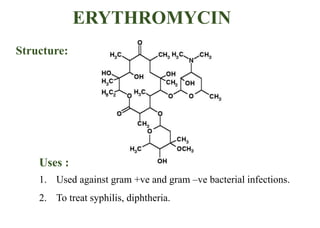
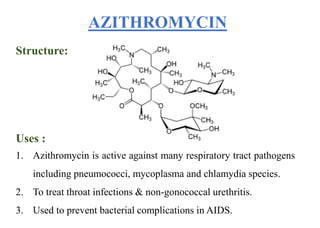
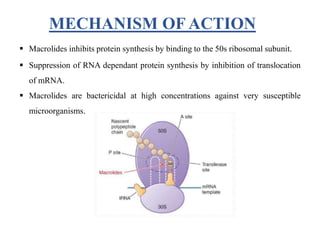
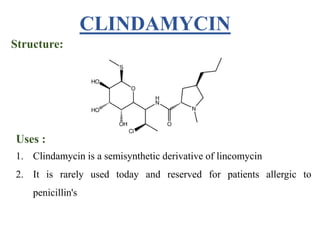
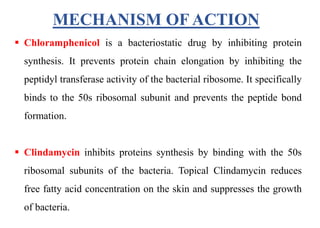
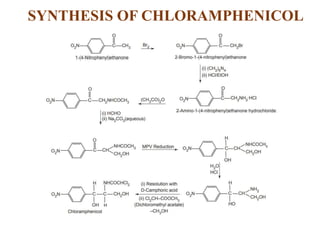
This document provides information about antibiotics, including their history and classifications. It begins with definitions of antibiotics and discusses their discovery, including the discovery of penicillin by Alexander Fleming in 1928. It then classifies antibiotics based on their spectrum of activity (narrow vs broad), source (natural, semisynthetic, synthetic), mechanism of action, and chemical structure. Specific examples are given for different classes. The remainder discusses various penicillins and cephalosporins, providing their structures and uses for treating bacterial infections.