Assessment of microwave assisted and hydrodistllation extraction on Echinops persicus essential oils chemical composition and evaluation of its biological activity
- 1. ARTICLE TMR | September 2019 | vol. 4 | no. 5 |246 Submit a manuscript: https://www.tmrjournals.com/tmr doi: 10.12032/TMR20190826132 Persian Medicine Assessment of microwave assisted and hydrodistllation extraction on Echinops persicus essential oils chemical composition and evaluation of its biological activity Maryam Soori1 , Hossein Abbaspour1 , Hamid Hashemi-Moghaddam2 * 1 Department of Biology, Damghan Branch, Islamic Azad University, Damghan 3671639998, Iran. 2 Department of Chemistry, Damghan Branch, Islamic Azad University, Damghan 3671639998, Iran. *Corresponding to: Hamid Hashemi-Moghaddam, Department of Chemistry, Basic Science Faculty, Damghan Branch, Islamic Azad University, Cheshme ali BLVD, Damghan 3671639998, Iran. Email: h.hashemimoghadam@damghaniau.ac.ir, hashemimoghaddam@yahoo.com. Highlights Microwave assisted hydrodistillation method can extract more compounds and yield of essential oils from Echinops persicus than conventional hydrothermal method as well as further confirms that the methanol extract of E. persicus plant exhibits considerable antioxidant and antimicrobial properties. Traditionality E. persicus is well-known as Shakarook in local Persian botany and it has been known since the time of Avicenna and was prescribed for cough and respiratory system as an effective drug. In Persian folk medicine, this plant has been widely prescribed as a flavoring agent and an effective remedy to treat influenza, cough, fever, throat dryness, etc. E. echinatus is also extensively utilized in Indian traditional folklore and Pakistan ethno-veterinary medicine.
- 2. ARTICLE TMR | September 2019 | vol. 4 | no. 5 |247 Submit a manuscript: https://www.tmrjournals.com/tmr doi: 10.12032/TMR20190826132 Abstract Background: E. persicus which is well-known as Shakarook in local Persian botany and is extensively utilized in different parts of in Iran. Materials and methods: Essential oils from the aerial parts of Echinops persicus were isolated using hydrodistillation (HD) and microwave assisted hydrodistillation (MAHD) methods and the respective chemical profiles were analyzed by means of GC-MS technique. The in vitro antioxidant and antimicrobial activities of methanol extracts of E. persicus were investigated via using 2,2'-diphenyl-1-picrylhydrazyl (DPPH) assay as well as agar well-diffusion methods. The minimun inhibitory concentrations (MICs) of the methanol extracts of E. persicus against the test microorganisms were determined by the broth microdilution method. Results: GC-MS essential oils analysis shows 29 and 36 compounds constituting 91.9% and 98.2% of the total oils using HD and MAHD methods, respectively. Furthermore, the methanol extracts of E. persicus exhibited higher DPPH radical scavenging activity than vitamin C with an IC50 value of 0.42 ± 0.16 µg/mL. Moreover, the prepared methanol extracts preliminarily showed promising antimicrobial activities against S. aureus with the MIC value of 6.2 mg/mL. Conclusion: This study confirms that the methanol extract of E. persicus plant exhibits considerable antioxidant and antimicrobial properties in vitro. Keywords: Echinops persicus, Essential oil, Methanol extracts, Antioxidation, Antimicrobial. Acknowledgments: Financial and technical supports from the office for research affair of Islamic Azad University, Damghan Branch are gratefully acknowledged. Abbreviations: HD, Hydrodistillation; MAHD, Microwave assisted hydrodistillation; MIC, Minimun inhibitory concentration; DPPH, 2,2'-Diphenyl-1-picrylhydrazyl; NFT, Nitrofurantoin; NA, Nalidixic acid; MHA, Mueller-Hinton agar; FID, Flame ionization detector; CLSI, Clinical and Laboratory Standards Institute; OM, Oxygenated monoterpenes; SH, Sesquiterpene hydrocarbons; OS, Oxygenated sesquiterpenes; NH, Non-terpene hydrocarbons, DH: Diterpene hydrocarbons; OD: Oxygenated diterpenes; MH: Monoterpene hydrocarbon; RE: Rutin equivalent. Competing interests: The authors declare that there is no conflict of interest. Citation: Maryam Soori, Hossein Abbaspour, Hamid Hashemi-Moghaddam. Assessment of microwave assisted and hydrodistllation extraction on Echinops persicus essential oils chemical composition and evaluation of its biological activity. Traditional Medicine Research 2019, 4 (5): 246-256. Executive Editor: Nuo-Xi Pi. Submitted: 17 May 2019, Accepted: 26 August 2019, Online: 5 September 2019.
- 3. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |248 doi: 10.12032/TMR20190826132 Background Echinops is a famous genus from the Asteraceae family consisting of about 120 species of flowering plants [1]. It is usually known as globe thistles. The plants belonging to this genus frequently appear as spiny foliage leading to spherical flower heads in nature [2]. E. persicus which is well-known as Shakarook in local Persian botany and it has been known since the time of Avicenna and was prescribed for cough and respiratory system as an effective drug [3]. In Persian folk medicine, this plant has been widely prescribed as a flavoring agent and an effective remedy to treat influenza, lung disease, asthma, constipation, depression, overweight, bellyache, cough, fever, as well as throat dryness [4-8]. It also possesses strong laxative, expectorant and anti-cancer impacts [9-10]. Moreover, a balancing role in the digestive system of the human body has been attributed to this medicinal species [5]. In Indian traditional folklore usage, E. echinatus is extensively utilized as a potent drug to cure sexual debility [11]. In Pakistan ethno-veterinary medicine, E. echinatus has been recommended as a tonic for camels [12]. Diverse pharmacological, biological, and phytochemical reports on different Echinops species have been reported in the literature involving antihelmentic [13], antimalarial [14], anti-inflammatory [15-17], larvicidal [18], diuretic [11], insecticidal [19], analgesic [11], reproductive [11], antiproliferative [20], antifungal [21-25], antioxidant [26], hepatoprotective [11], cytotoxic [27-29], anti-ulcer [30], antifeedant [29], immunomodulatory [4], antiplasmodial [31], antibacterial [25, 32, 33], toxicological [31], anti-irritant [34], antipyretic [11, 35], anti-worm [25], wound healing [36] and antimicrobial [37-39] activities. As there are several activities on Echinops genus, developing more effective extraction techniques are required which was secure and agreeable to automation. Today, microwave heating there has been a growing interest because of its speed, also it is an environment friendly technology for the extraction of a wide array of samples [40]. The combination of microwave-assisted extraction and hydrodistillation (HD) has been effectively applied to extraction essential oils from different plants [41-46]. More effective and selective heating, time efficiency, faster response to process heating control are advantages microwave assisted hydrodistillation (MAHD) extraction compared with conventional extraction methods [40, 42]. In this work, essential oil was extracted from the aerial parts of E. persicus by using HD and MAHD methods for the first time. Separation and identification of the constituents of the chemical profiles have been made using GC and GC-MS approaches. Furthermore, antioxidant activity of the plant methanol extract was assessed using the 2,2'-diphenyl-1-picrylhydrazyl (DPPH) assay while its antimicrobial properties were examined using broth microdilution and agar well diffusion methods. Experimental Chemicals 2,6-Ditertbutyl-4-methylphenol (butylated hydroxytoluene) standard antioxidant agent, nitrofurantoin (NFT) antibiotic, nalidixic acid (NA) antibiotic and DPPH were all purchased from Sigma-Aldrich GmbH (Munich, Germany). The Mueller-Hinton agar (MHA) and MHB (Muller Hinton broth) culture media were purchased from Merck Company, Germany. Standard strains: Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922) were provided by Iran Medical Sciences University, Tehran, Iran. Plant material E. persicus was harvested in the late of April 2016 in the agricultural research farm, Hamadan Province, Hamadan, Iran. The plant material was first characterized by Dr Bostan Rodi botanist in the Damghan Branch, Islamic Azad University and for further authentication the whole plant material was deposited at the Research Institutes of Forest Herbarium and Rangelands (No: 4123451). For oil extraction, the aerial parts were separated and final step it dried in a clean place at shadow to avoid extra damaging and to lower unpleasant cross-contamination. HD of essential oil Commercially available clevenger apparatus (Ashk shishe Co.Tehran Iran) was used for HD. In a preliminary step, the E. persicus sample was dried for one week at shade and then it was precisely weighed. In order to hydrate its external layers, 50 g of plant was immersed in 500 mL water, then volatile oils were extracted at successive times. Each experiment was carried out twice. The optimized distillation time was 3 h. Finally, the oil was dehydrated with anhydrous sodium sulfate, it was separated and capped under nitrogen and kept in refrigerator at -4 °C. MAHD of essential oil The microwave oven (Samsung, South Korea) used for performance of the MAHD was operated at a safe and commonly used frequency of 2450 MHz. This oven had an interior cavity with the general dimensions of 29 cm × 37 cm × 40 cm which was capable of providing of maximum powers equal to 1000 W within
- 4. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |249 doi: 10.12032/TMR20190826132 the experiments. In our proposed MAHD methodology, a proper hole was first drilled on the top of the microwave. Immediately after, a flat-bottom flask (1000 mL) was situated in the cavity of the microwave assemble and directly connected to the circulatory Clevenger via the provided hole [47-49]. To avoid scattering of microwave beams in the laboratory environment, around the hole was completely closed with a layer of an aluminum foil. To perform the MAHD approach, fifty g portions of the plant were first immersed in 0.5 L of distilled water at 25 °C under the atmospheric pressure for 1 h. Immediately after, the excess water was drained off. The required time for the soaking step was determined through our primary evaluations. In the next step, the moistened plant material was placed in a flat-bottom flask of the system assembly. Throughout the extraction, the vapor constantly passed through the condenser. Time of extraction was optimized, as in long times the extraction yield was reduced. For each case, experiments were replicated twice. The storage procedure for the essential oil obtained by the MAHD approach was the same as described for the water-distilled oil. Preparation of the methanol extracts of E. persicus In the first step, 50 g the aerial parts of plant (E. persicus) were cut up and crushed in a coffee mill (Moulinex Corporation, France). Obtained powder was transferred to a darkcolored flasks, mixing with 85% (v/v) methanol at a ratio of 15:100 (m/v plant material to-solvent) and heating at 50 °C for 35 min. Subsequently, the slurry was filtered through Whatman No. 1 filter paper and the resulting deposit was extracted twice and supernatants were combined and evaporated under vacuum using a rotary vapor (IKA, Germany) at 40 °C. Finally, the extracts were stored in refrigerator at 4 °C. GC and GC-MS Analysis Quantitative and qualitative assessments of the extracted oils were carried out using GC and GC-MS instruments. A gas chromatograph (Varian 3800, Australia) was used for quantitative analyses; experimental conditions were chosen as follow: injector temperature 290 °C with the split ratio 1:10 and a FID (flame ionization detector) temperature 250 °C. N2 at flow rate of 0.8 mL/min was used as carrier gas. Capillary column was CP-Sil 5 CB (30 m × 0.25 mm × 0.25 μm film thickness). The oven temperature was held at 50 °C for 5 min and heated to 240 °C at a rate of 3 °C/min followed by an extra rise to 300 °C with a programmed 5 °C/min ramp. Three min final hold was applied for a clean-up of column. Quantitative data were obtained through the system area percentage. GC-MS determinations were performed on an HP-6890 GC system coupled with a 5973-network mass selective detector, and equipped with an HP-5MS capillary fused silica column (30 m × 0.25 mm I.D. × 0.32 μm film thickness). The working conditions were the same as described above but the carrier gas was He. Mass spectra were taken at an ionization voltage of 70 eV and were recorded over the m/z range 20-500 amu. Chromatographic measurements repeated three times, and the mean of the retention indices as well as the mean percentage of each component were considered. Duplicate times were not taken into consideration if they had differences higher than 1 s. Identification and quantification of essential oil ingredients Characterization and recognition of the constituent components of the essential oils from the aerial parts of E. persicus were made by: (1) exhaustive comparison of the mass spectral fragmentation pattern with respect to the authentic samples and the Kovat's retention indices (RI) relative to normal n-alkanes (C9-C25) with the authentic data tabulated in the literature [50] and a homemade software based upon our previously published papers in the literature; (2) the mass spectral archive available in the library (Wiley 275) of the GC-MS instrument; (3) matching of the fragmentation pattern of each constituent in each characterized profile with those available in National Institute of Standards and Technology Mass Spectral Library package with a resemblance percentage above 90%. The relative content of each constituent was evaluated regarding the related peak area through summing of all of the peaks (%). In this regard, no correction factor was used in the calculation process. In the last step, distinct volumes of the essential oils were injected onto the injection port of the GC-MS column, separately. Determination of total phenolic contents A proposed method that based on using the Folin-Ciocalteu reagent is utilized to assess the amount of total phenolic [51]. One hundred μL portions of methanol extracts from the aerial parts of E. persicus were transferred into the test tubes, followed with 0.75 mL of Folin-Ciocalteu reagent, which was previously diluted 10 times with deionized water. This solution was mixed gently and allowed to stand at 25 °C for 5 min. Then 0.75 mL of sodium carbonate (6.0% w/v) was added to the mixture and stirred with other reactants. Ninety min later, the absorbance was determined at 725 nm using the double beam UV-Vis spectrophotometer (Varian Cary 50, Australia). All of the determinations were performed in triplicate. Determination of total flavonoid contents The contents of total flavonoids in the methanol extracts from the aerial parts of E. persicus were assessed with a spectrophotometric method reported by Zhishen et al. [52] with some modification. Rutin was
- 5. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |250 doi: 10.12032/TMR20190826132 used as the standard. One mL of the prepared extract was diluted with an aqueous-ethanol solution (40.0% v/v) to the final volume of 5 mL, followed by 0.3 mL of an aqueous solution of NaNO2 (1:20, w/v), and then after 5 minutes followed by addition of 0.3 mL Al(NO3)3 (1:10, w/v). After being mixed and standing for 6 min, the resulting solution was added with 2.0 mL of NaOH (1.0 M) and then brought up to 10 mL by an aqueous ethanol (40% v/v). The mixed solution was incubated at 25 °C for 10 min. A spectrophotometer (UV2550, Shimadzu, Japan) using quartz cuvettes (10 ×10 mm) were used to measure its absorbance at 510 nm. The solution containing all the reagents except for the plant extract in an aqueous ethanol medium was used as the blank solution. All of the determinations were performed in triplicate. Determination of antioxidant activity by the DPPH method Based on the method of Onder et al. [50] with some modifications, the capability to scavenge DDPH free radicals was used to evaluate the in vitro antioxidant activities of methanol extracts from the aerial parts of E. persicus. The methanolic extracts and vitamin C, both at the concentration of 0.2 mg/L, were prepared and were separately to react with 2 mL of a methanolic solution of DPPH (0.004 g in 100 mL of methanol), vitamin C was used as positive control while DPPH without test samples was used as blank. Each experiment was performed 3 times. Then the plate was incubated in dark at 25 °C for 60 min and the absorbance was subsequently measured at 517 nm using a microplate reader (Biotek Epoch, USA). The ability to scavenge the DPPH free radical was calculated using the following equation [53]. DPPH scavenging effect (%) = (Ao-A1)/Ao × 100. Where Ao and A1 respectively imply the absorbance of the control (blank) and the absorbance in the presence of sample (E. persicus extract). Furthermore, the antioxidant activities of the plant extract was evaluated using the ascorbic acid standard curve and its corresponding activities were expressed as IC50 (mg/L). Antimicrobial activity by the agar well diffusion method The methanolic extract of was weighed and dissolved in phosphate buffer saline (PBS; pH 7.0-7.2) and DMSO at the concentration of 10 mg/mL, respectively and filtered through a 0.45 µm membrane filter. Staphylococcus aureus and Escherichia coli was suspended in sterile saline at the density of 1 × 106 /mL and inoculated onto the surface of MHA. The wells with 8 mm in diameter were cut from the agar and 0.06 mL of methanol extract solution at the concentration of 10 mg/L was then delivered into each well. NFT100 antibiotic and NA30 antibiotic were used as positive control for Staphylococcus aureus and Escherichia coli respectively. After 24 h incubation at 37 °C, all plates were examined for any zones of growth inhibition, and the diameters of these zones were measured in mm [54]. All the tests were performed in triplicate. Determination of minimum inhibitory concentration A broth microdilution susceptibility assay recommended by Clinical and Laboratory Standards Institute (CLSI), formerly known as NCCLS, was used to determine the MIC [54-55]. All the tests supplemented with Tween 80 detergent to a final concentration of 0.5% (v/v) were performed in Mueller Hinton Broth (MHB). The bacterial strains were cultured overnight at 37 ºC in MHA. Test strains were suspended in MHB at a final density of 5 × 105 /mL. Geometric dilutions of the methanol extract, with the range from 0.036 to 72.0 mg/mL, were prepared in a 96-well microtitre plate. MHB + Tween 80 was used as the growth control, MHB + Tween 80 + test oil was used as the sterility control. Plates were then incubated at 37 ºC for 24 h and the bacterial growth was confirmed. Statistical analysis All experiments were done in triplicate, and results were presented as mean ± SD. Statistical analysis was performed on the data by SPSS 11.0. Differences in inhibition zone were statistically analyzed by Student's t test. Values of P < 0.05 were considered significant. Results The yield of essential oils with HD and MAHD methods The yield of extraction was 0.25 % and 43% (w/w) for respectively (weight of the collected oil per g of dried plant). The comparison of obtained results for yield of essential oils extraction by these two methods show apparent increase in yield by MAHD method. Chemical profiles of the volatile oils of E. persicus by HD and MAHD Characterization of extracted essential oils of E. persicus show that its ingredients mainly consisting of oxygenated monoterpenes (OM) by the aid of the described HD and MAHD techniques. Identificated compounds are several OM, sesquiterpene hydrocarbons (SH), oxygenated sesquiterpenes (OS), non-terpene hydrocarbons (NH), diterpene hydrocarbons (DH) and oxygenated diterpenes (OD). Notably, no monoterpene hydrocarbon (MH) contributed to the recognized profiles of the analyzed essential oils. Chromatographic information of the volatile oils obtained from the E. persicus by the above-mentioned methods are tabulated in Table 1. There are satisfactory agreements between the calculated numerical values of Kovats retention indices
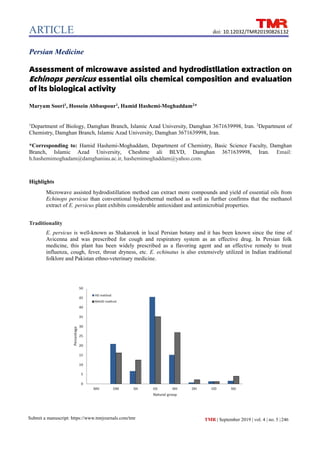
- 6. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |251 doi: 10.12032/TMR20190826132 and those cited in the literature. In Figure 1, The component classes found in the aerial parts oils in E. persicus L. by HD and MAHD methods. Table 1 The essential oils chemical compositions from aerial parts of Echinops persicus obtained using the HD and MAHD methods Number Compound Class KI (Cal.) KI (Lit.) RT HD RT MAHD Characterization criteria 1 n-Undecane NH 1100.1 1100 1.2 0.5 RI, MS, LRR 2 Borneol OM 1167.1 1165 0.7 0.9 RI, MS 3 Isomenthol OM 1181.2 1182 0.6 1.1 RI, MS 4 ND - 1190.7 - - 0.6 RI, MS, LRR 5 trans-4-Caranone OM 1195.5 1196 1.4 0.9 RI, MS, LRR 6 Methyl α-Cyclogeranate OM 1199.1 1197 1.8 1.3 RI, MS, LRR 7 Dodecane NH 1200.4 1200 4.0 4.4 RI, MS, LRR 8 cis-4-Caranone OM 1201.2 1200 1.3 1.2 RI, MS, LRR 9 Fenchyl acetate OM 1221.3 1221 7.0 4.8 RI, MS, LRR 10 β-Cyclocitral OM 1220.6 1219 2.5 1.9 RI, MS, LRR 11 Isobornyl formate OM 1238.8 1239 5.6 4.1 RI, MS, LRR 12 ND - 1389.4 - 1.6 0.4 RI, MS, LRR 13 n-Tetradecane NH 1398.5 1400 - 0.9 RI, MS 14 trans-Caryophyllene SH 1421.3 1417 2.0 2.8 RI, MS, LRR 15 Aromadendrene SH 1440.2 1439 - 1.2 RI, MS, LRR 16 Germacrene D SH 1485.1 1484 0.7 1.0 RI, MS, LRR 17 β-Dihydro agarofuran OS 1504 1503 2.0 1.4 RI, MS, LRR 18 β-Bisabolene SH 1507.3 1505 1.3 2.2 RI, MS, LRR 19 γ-Cadinene SH 1516.5 1513 1.4 2.4 RI, MS 20 δ-Cadinene SH 1527.5 1522 1.3 2.9 RI, MS, LRR 21 Elemol OS 1524.5 1548 0.6 0.7 RI, MS, LRR 22 Caryophyllene oxide OS 1550.1 1582 1.0 1.7 RI, MS, LRR 23 Hexadecane NH 1599.4 1600 6.0 1.6 RI, MS, LRR 24 Guaiol OS 1601.3 1600 - 4.6 RI, MS, LRR 25 γ-Eudesmol OS 1629.4 1630 16.7 12.1 RI, MS, LRR 26 7-epi-α-Eudesmol OS 1658.1 1662 1.4 0.9 RI, MS, LRR 27 Hinesol OS 1638 1640 5.7 7.0 RI, MS, LRR 28 Valerianol OS 1657.1 1656 9.5 5.9 RI, MS, LRR Pogostol OS 1651.2 1651 8.6 0.9 RI, MS, LRR 30 Heptadecane NH 1699.9 1700 - 1.5 RI, MS, LRR 31 n-Octadecane NH 1800.4 1800 1.8 1.5 RI, MS, LRR 32 Beyerene DH 1933.1 1931 0.7 2.2 RI, MS, LRR 33 ND - 1804.1 - - 0.7 RI, MS 34 n-Eicosane NH 2001.2 2000 1.0 1.4 RI, MS, LRR 35 ND - 1947.1 - - 0.6 RI, MS, LRR 36 Incensole acetate OD 2184.3 2184 - 1.2 RI, MS, LRR 37 ND - 2198.3 - 1.3 0.6 RI, MS, LRR 38 ND - 2201.1 - - 0.5 RI, MS,
- 7. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |252 doi: 10.12032/TMR20190826132 Table 1 The essential oils chemical compositions from aerial parts of Echinops persicus obtained using the HD and MAHD methods (Continued) 39 n-Docosane NH 2219.9 2200 1.2 0.7 RI, MS, LRR 40 n-Tricosane NH 2302.1 2300 - 0.7 RI, MS, LRR 41 Libocedrol NH 2343.1 2344 - 3.2 RI, MS, LRR 42 ND - - - - 0.6 RI, MS 43 n-Tetracosane NH 2398.7 2400 - 10.5 RI, MS, LRR Total 91.9 98.2 KI (Cal.), Kovats retention index calculated with respect to n-alkenes on a HP-5MS capillary column; KI (Lit.), Kovats retention index given in literature; RT, Retention time; HD, Hydrodistillation; MAHD, Microwave assisted hydrodistillation; ND: Not detected; NH: Non-terpene hydrocarbons; OM: Oxygenated monoterpens; SH: Sesquiterpene hydrocarbons; OS: Oxygenated sesquiterpens; DH: Diterpene hydrocarbons; OD: Oxygenated diterpenes; RI: Retention index, MS: Mass spectra, LRR: Laboratory resemblance report ( > 90%). Figure 1 The component classes in the aerial parts of the E. persicus L. oils by HD and MAHD methods HD, Hydrodistillation; MAHD, Microwave assisted hydrodistillation; MH: Monoterpene hydrocarbons; OM: Oxygenated monoterpens; SH: Sesquiterpene hydrocarbons; OS: Oxygenated sesquiterpens; NH: Non-terpene compounds; DH: Diterpene hydrocarbons; OD: Oxygenated diterpenes; ND: Not detected. Table 2 Methanolic extracts antimicrobial activity of the E. persicus L Note: a: according to CLSI 2013 (Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement, Document M100-S20. Wayne, PA: Clinical and Laboratory Standards Institute 2013; 22-50 [51].). b: Compared with NA30, P < 0.05. NFT: Nitrofurantoin;NA, Nalidixic acid. Bacteria Diameter of inhibition zone (mm) Positive control Methanolic extracts Escherichia coli 25 ±1.7 (NA30, standdrad range: 23-28 mm [51]) 18 ± 1.5 b Staphylococcus aureus 21 ±1.1 (NFT100, standdrad range: 20-25 mm [51]) 22 ± 0.8
- 8. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |253 doi: 10.12032/TMR20190826132 In the HD extraction method (Table 1), 29 components could be identified in the oil from the aerial parts: eight OM (20.9%), five SH (6.7%), eight OS (45.5%), six NH (15.2%), one diterpene hydrocarbon (0.7%), and one oxygenated diterpene (1.3%). Specifically, in the hydrodistilled oil from the plant aerial parts γ-eudesmol (16.7%) was found to be the major constituent followed by valerianol (9.5%), pogostol (8.6%), fenchyl acetate (7.0%), hexadecane (6.0%), hinesol (5.7%) and isobornyl formate (5.6%). On the other hand, in the essential oil was extracted by MAHD method, 36 volatile components were identified in the aerial parts oils using. The main components were γ-eudesmol (12.1%), hinesol (7.0%), valerianol (5.9%), fenchyl acetate (4.8%), guaiol (4.6%) and dodecane (4.4%). In terms of general categories, eight OM (16.2%), six SH (12.5%), nine OS (35.2%), eleven NH (26.9%), one diterpene hydrocarbon (2.2%) and one oxygenated diterpene (1.2%) were identified in the essential oil of the plant sample (E. persicus) obtained by the MAHD method. Total phenolic and flavonoids contents To assess the total phenolic levels in the organic extract of the plant material, a standard calibration curve (0.01-0.05 mg mL-1 ) was plotted using gallic acid (r2 = 0.99). The total phenolic content was finally calculated as gallic acid equivalents in milligram per 100 g of plant extract. Accordingly, the contents of total phenolic were found to be 99.22 (mg gallic acid equivalents/g dry leaves). On the other hand, in the determination of the flavonoid contents in the methanol extract of E. persicus, a standard curve equation obtained having an equation of A = 1.971 × C, R2 = 0.9986 where A was the absorbance and C was the rutin equivalent (RE) mg/mL concentration. The total flavonoids content in the extracts was calculated and expressed as mg RE per g dry weight. In our study, total flavonoids contents in the methanol extracts from the aerial parts of E. persicus were found to be 17.5 ± 0.50 mg RE/g dry weight. Antioxidant activity results A methanolic solution of the stable free radical, DPPH was used for the radical-scavenging activity of the prepared plant extracts. The obtained results showed that IC50 value for the methanolic extracts from the of E. persicus and vitamin C were 0.42 ± 0.16 and 0.55 ± 1.8 µg/mL, respectively. This implies that the extracts of this plant possess a very strong antioxidant activity even more than vitamin C as a powerful antioxidant agent. Antimicrobial activity In this study, methanolic extract from of the E. persicus was evaluated for exploration of possible antimicrobial activity against certain bacterial strains, which are frequently regarded as important human pathogens. Susceptibility of the plant extracts was evaluated by the agar well diffusion method. In our experiments, the methanolic extract of the E. persicus showed strong bactericidal effect against all the tested microorganisms involving both Gram positive and Gram-negative bacterial strains (Staphylococcus aureus and Escherichia coli) (Table 2). According to the result of broth microdilution susceptibility assay, the extract isolated from the aerial parts of E. persicus was found to be sensitive against Staphylococcus aureus microorganism with a minimum inhibitory concentration (MIC) value of 6.2 mg/mL. However, MIC of this extract against Escherichia coli was not detected and further studies need to be undertaken to elucidate its exact antimicrobial activities. Discussion In this project, the essential oils from the aerial parts of E. persicus were successfully isolated by using HD and MAHD methods and were subsequently characterized using the GC-MS instrumentation for the first time. Comparative assessment of various classes of the oil ingredient show that in extracted oils by different methods, OS established the main ingredient and the second major class are non-terpene and OM when using the proposed HD and MAHD methodologies. Regarding our characterization, γ-eudesmol was the most frequent natural compound occurring in the two identified profiles. Weyerstahl et al. have analyzed strong patchouli-like and woody smelling essential oil of the rhizomes of E. giganteus var. Ielyi C. D. Adams (Compositae) and reported that all the chemical profile consisted of sesquiterpenes, which were mainly due to triquinane compounds [56]. In this attempt, three new tricyclic sesquiterpene skeletons, namely cameroonane, prenopsane and nopsane naturally occurring have been identified for the first time. Furthermore, a biogenetic pathway from presilphiperfolane cation C to the cameroonane K, prenopsane L and nopsane M cations has been shown. In accordance with this work, cameroonanol and prenopsanol were recognized as the main contributors to the fragrance of the total oil. Also, in the work of Liu et al. on the essential oil from the aerial parts of E. latifolius Tausch, high prevalence of monoterpenoids (78.45%) was reviled by GC-MC analysis [19]. In accordance with this study, the respective chemical profile was characterized by high quantities of 1,8-cineole (19.6%), (Z)-β-ocimene (18.4%), β-pinene (15.6%), β-myrcene (4.8%) and carvone (4.4%). Finally, Radulovic and Denic have analyzed the essential oils from the roots of E. bannaticus Rochel ex Schrad. and E. sphaerocephalus L. (Asteraceae) from Serbia [57]. In this report, the oils were characterized by high contents of two relatively rare groups of compounds: S-containing polyacetylene
- 9. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |254 doi: 10.12032/TMR20190826132 compounds (65.5 and 64.1%, respectively) and triquinane sesquiterpenoids (12.7 and 20.9%, respectively). The findings given in this work highlighted that several compounds belonging to S-containing polyacetylene and triquinane sesquiterpenoids represent metabolites which were novel for the analyzed Echinops species or even the whole genus. A simple comparison on the obtained results using the utilized approaches (HD and MAHD) reveals that considering the most important class of natural compounds constituting that concerned chemical profiles, there are some similarities between our findings and the previously published papers [58-59]. However, there are apparent differences between the recognized chemical profiles in the present report and some of the other previous attempts dealing with the characterization of the chemical composition of the essential oils from other species of the Echinops genus [19, 56, 57, 59, 60]. The plant extracts as antioxidant and antimicrobial agents always show two main features: natural origin is the first which earnings more safety to the public and the environment, lower risk for resistance development through pathogenic microorganisms is the second characteristic. About of its biological activity, in the literature, a variety of studies have been performed on the extracts of various plants to screen the concerned antimicrobial activity and/or to explore novel antimicrobial compounds. Therefore, medicinal plants are finding their way into pharmaceuticals, nutraceuticals and food supplements disciplines [61-63]. In short, based on our analysis, the extract from the aerial parts of E. persicus was observed to against Gram-positive microorganism Staphylococcus aureus with a MIC value of 6.2 mg/mL. However, MIC of this extract against Escherichia coli as a Gram-negative microorganism was not detected and further studies should be undertaken to elucidate its exact antimicrobial activities. Conclusion A total of 29-36 compounds were identified in the related profiles covering 91.9-98.2% of their compositions in the separated essential oils from the aerial parts of E. persicus using the HD and MAHD approches, respectively. In addition, the methanolic extract of E. persicus was observed to have the definite antimicrobial activities against S. aureus microorganism, to be suggested as a new potential source of natural antioxidants and antimicrobial agents for pharmaceutical and food industries. References 1. Brenzel KN. Sunset Western Garden Book. Sunset Books, 2001. 2. Dorling K. RHS AZ Encyclopedia of Garden Plants. Dorling Kindersley Ltd, 2008. 3. Nasirzade A, Javidtash E, Riasat M. Echinops percicus species and some of the biological characteristics weevil in Fars province (Larinus vulpes Oliv.) manna generation. Quarterly Res Med Aromat Plants 2005; 21: 335-336. 4. Hamedi A, Farjadian S, Karami MR. Immunomodulatory properties of Trehala manna decoction and its isolated carbohydrate macromolecules. J Ethnopharmacol 2015; 162: 121-126. 5. Omidbaigi R. Production and Processing of Medicinal Plants. 6th ed. Tehran: Behnashr, Astan Ghods Razavi Press, 2012. (Persian) 6. Zargari A. Medicinal Plants. Tehran, Iran: Tehran University Publication, 1996. (Persian) 7. Mozaffarian V. A. Dictionary of Iranian Plant Names. Iran: Farhang Moaser Press, 1996. (Persian) 8. Mohseni S, Sani AM, Tavakoli M, et al. Effect of extraction conditions on antioxidant activities of Echinops persicus. J Essent Oil-Bear Plants 2017; 20: 1633-1644. 9. Ghasemi A. Medicinal and Fragrant Plants, Identification and Evaluation of Their Effects. Shahrekord, Iran: Islamic Azad University, Shahrekord Press, 2009. (Persian) 10. Mozaffarian V. Identification of the Iranian Medicinal and Fragrant Plants Tehran, Iran: Farhang Moaser Press, 2012. (Persian) 11. Maurya SK, Kushwaha AK, Seth A. Ethnomedicinal review of Usnakantaka (Echinops echinatus Roxb.). Pharmacogn Rev 2015; 9: 149-154. 12. Saeed Khattak N, Nouroz F, Ur Rahman I, et al. Ethno veterinary uses of medicinal plants of district Karak, Pakistan. J Ethnopharmacol 2015; 171: 273-279. 13. Hussien J, Urgessa K, Regassa F, et al. Antihelmentic effects of the essential oil extracts of selected medicinal plants against Haemonchus contortus. Int J Agric Res 2011; 6: 290-298. 14. Sathiyamoorthy P, Lugasi-Evgi H, Schlesinger P, et al. Screening for cytotoxic and antimalarial activities in desert plants of the Negev and Bedouin market plant products. Pharm Biol 1999; 37: 188-195. 15. Singh B, Gambhir SS, Pandey VB, et al. Anti-inflammatory activity of Echinops echinatus. J Ethnopharmacol 1989; 25: 189-199. 16. Yadava RN, Singh SK. New anti-inflammatory active flavanone glycoside from the Echinops echinatus Roxb. Indian J. Chem., Sect. B: Org. Chem Incl Med Chem 2006; 45:1004-1008. 17. Talhouk RS, Karam C, Fostok S, et al. Anti-inflammatory bioactivities in plant extracts.
- 10. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |255 doi: 10.12032/TMR20190826132 J Med Food. 2007;10:1-10. 18. Pavela R. Larvicidal effects of some Euro-Asiatic plants against Culex quinquefasciatus Say larvae (Diptera: Culicidae). Parasitol Res 2009; 105: 887-892. 19. Liu XC, Hao X, Zhou L, et al. GC-MS analysis of insecticidal essential oil of aerial parts of Echinops latifolius Tausch. J Chem 2013. 20. Csupor-Löffler B, Hajdú Z, Réthy B, et al. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part II. Phytother Res 2009; 23: 1109-1115. 21. Dzoyem JP, Tchuenguem RT, Kuiate JR, et al. In vitro and in vivo antifungal activities of selected Cameroonian dietary spices. BMC Complement. Altern Med 2014; 14. 22. Bahraminejad S, Abbasi S, Fazlali M. In vitro antifungal activity of 63 Iranian plant species against three different plant pathogenic fungi. Afr J Biotechnol 2011; 10: 16193-16201. 23. Shahidi-Bonjar GH, Aghighi S, Karimi Nik A. Antibacterial and antifungal survey in plants used in indigenous herbal-medicine of south east regions of Iran. J Biol Sci. 2004;4:405-412. 24. Fokialakis N, Cantrell CL, Duke SO, et al. Antifungal activity of thiophenes from Echinops ritro. J Agric Food Chem 2006; 54: 1651-1655. 25. Hymete A, Iversen TH, Rohloff J, et al. Screening of Echinops ellenbeckii and Echinops longisetus for biological activities and chemical constituents. Phytomedicine 2005; 12: 675-679. 26. Erenler R, Yilmaz S, Aksit H, et al. Antioxidant activities of chemical constituents isolated from Echinops orientalis Trauv. Rec Nat Prod 2014; 8: 32-36. 27. Ali MA, Abul Farah M, Al-Hemaid FM, et al. In vitro cytotoxicity screening of wild plant extracts from Saudi Arabia on human breast adenocarcinoma cells. Gene Mol Res. 2014; 13: 3981-3990. 28. Kuete V, Sandjo LP, Wiench B, et al. Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat Cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J Ethnopharmacol 2013; 149: 245-253. 29. Fokialakis N, Osbrink WLA, Mamonov LK, et al. Antifeedant and toxicity effects of thiophenes from four Elchinops species against the Formosan subterranean termite, Coptotermes formosanus. Pest Manag Sci. 2006; 62: 832-838. 30. Asadi-Rad A, Najafzadeh-Varzi H, Farajzadeh-Sheikh A. Evaluation of anti-ulcer activity of Echinops persicus on experimental gastric ulcer models in rats. Vet Res Forum. 2010; 1: 188-191. 31. Toma A, Deyno S, Fikru A, et al. In vivo antiplasmodial and toxicological effect of crude ethanol extract of Echinops kebericho traditionally used in treatment of malaria in Ethiopia Malaria J 2015; 14. 32. Shahidi Bonjar GH, Nik AK, Heydari MR, et al. Anti-pseudomona and anti-bacilli activity of some medicinal plants of Iran. Daru 2003; 11: 157-163. 33. Tekwu EM, Askun T, Kuete V, et al. Antibacterial activity of selected Cameroonian dietary spices ethno-medically used against strains of Mycobacterium tuberculosis. J Ethnopharmacol 2012; 142: 374-382. 34. Zaheer M, Hussain N, Rahman S, et al. Anti irritant activity of extract from the aerial parts of Echinops echinatus Compositae. Pakistan J Sci Indus Rese Series B: Biol Sci 2012; 55: 40-45. 35. Alam MK, Ahmed S, Anjum S, et al. Evaluation of antipyretic activity of some medicinal plants from Cholistan desert Pakistan. Pak J Pharma Sci. 2016; 29: 529-533. 36. Jagadish NRN, Mohmood R. Wound healing effect of Echinops echinatus Roxb. roots. Indian Drugs 2009; 46: 342-346. 37. Aldoweriej AM, Alharbi KB, Saeed EMA, et al. Antimicrobial activity of various extracts from some plants native to Alqassim Region, Saudi Arabia. J Food Agric Environ 2016; 14: 14-19. 38. Toroglu S, Keskin D, Vural C, et al. Comparison of antimicrobial activity of Echinops viscosus Subsp Bithynicus and E. microcephalus leaves and flowers extracts from Turkey. Int J Agric Biol. 2012; 14: 637-640. 39. Savita Sharma KM, Mehta BK. Antimicrobial activity of Echinops echinatus root extracts. Fitoterapia 1989; 60: 82-83. 40. Ganzler K, Salgo A, Valko K. Microwave extraction: a novel sample preparation method for chromatography. J Chromatogr 1986; 371: 299-306. 41. Wang ZM, Ding L, Li TC, et al. Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L. and Zanthoxylum bungeanum Maxim. J Chromatogr. A 2006; 1102:11-17. 42. Wang HW, Liu YQ, Wei SL, et al. Comparison of microwave-assisted and conventional hydrodistillation in the extraction of essential oils from Mango (Mangifera indica L.) flowers. Molecules 2010; 15: 7715-7723. 43. Gholivand MB, Piryaei M, Abolghasemi MM, et al. Comparison of microwave-assisted headspace single-drop microextraction (MA-HS-SDME) with hydrodistillation for the determination of volatile compounds from Prangos uloptera. J Essent Oil Res 2013; 25: 49-54. 44. Liu YQ, Wang HW, Wei SL, et al. Chemical composition and antimicrobial activity of the essential oils extracted by microwave-assisted hydrodistillation from the flowers of two
- 11. ARTICLE Submit a manuscript: https://www.tmrjournals.com/tmr TMR | September 2019 | vol. 4 | no. 5 |256 doi: 10.12032/TMR20190826132 Plumeria species. Anal Lett 2012; 45: 2389-2397. 45. Thach LN, Nhung TH, My VTN, et al. The new rich source of rotundifolone: Mentha aquatica Linn. var. crispa oil from microwave-assisted hydrodistillation. J Essent Oil Res 2013; 25: 39-43. 46. Khazayi M, Afshari H, Hashemi-Moghaddam H. Evaluation of Extraction Method and Chemical Modifier on Chemical Composition of the Essential Oils from the Roots of Rosa canina L. J Essent Oil-Bear Plants 2019; 22: 131-140. 47. Mohammadhosseini M, Akbarzadeh A, Hashemi-Moghaddam H, et al. Chemical composition of the essential oils from the aerial parts of Artemisia sieberi by using conventional hydrodistillation and microwave assisted hydrodistillation: A comparative study. J Essent Oil-Bear Plants 2016; 19: 32-45. 48. Mohammadhosseini M, Akbarzadeh A, Hashemi-Moghaddam H. Gas chromatographic-mass spectrometric analysis of volatiles obtained by HS-SPME-GC-MS technique from Stachys lavandulifolia and evaluation for biological activity. J Essent Oil-Bear Plants. 2016; 19. 49. Hashemi-Moghaddam H, Mohammadhosseini M, Azizi Z. Impact of amine-and phenyl-functionalized magnetic nanoparticles impacts on microwave-assisted extraction of essential oils from root of Berberis integerrima Bunge. J Appl Res Med Aromat Plants 2018; 10:1-8. 50. Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. USA: Allured Pubishing Co., 2007. 51. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement, Document M100-S20. Wayne, PA: Clinical and Laboratory Standards Institute 2013; 22-50. 52. Vardar-Ünlü G, Candan F, Sökmen A, et al. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). J Agric Food Chem 2003; 51: 63-67. 53. Velioglu Y, Mazza G, Gao L, et al. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 1998; 46: 4113-4117. 54. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 1999; 64: 555-559. 55. Onder FC, Ay M, Sarker SD. Comparative study of antioxidant properties and total phenolic content of the extracts of Humulus lupulus L. and quantification of bioactive components by LC-MS/MS and GC-MS. J Agric Food Chem 2013; 61: 10498-10506. 56. Weyerstahl P, Marschall H, Seelmann I, et al. Cameroonane, prenopsane and nopsane, three new tricyclic sesquiterpene skeletons. Eur J Org Chem. 1998: 1205-1212. 57. Radulovic NS, Denic MS. Essential oils from the roots of Echinops bannaticus Rochel ex Schrad. and Echinops sphaerocephalus L. (Asteraceae): Chemotaxonomic and Biosynthetic Aspects. Chem Biodiv 2013; 10: 658-676. 58. Tariku Y, Hymete A, Hailu A, et al. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem Biodiv 2011; 8: 614-623. 59. Hymete A, Rohloff J, Iversen TH, et al. Volatile constituents of the roots of Echinops kebericho Mesfin. Flav Fragr J 2007; 22: 35-38. 60. Papadopoulou P, Couladis M, Tzakou O. Essential oil composition of two Greek Echinops species: Echinops graecus Miller and Echinops ritro L. J Essent Oil Res 2006; 18: 242-243. 61. Ji H, Kim H, Beuchat LR, et al. Synergistic antimicrobial activities of essential oil vapours against Penicillium corylophilum on a laboratory medium and beef jerky. Int J Food Microbiol 2019; 291: 104-110. 62. Correa MS, Schwambach J, Mann MB, et al. Antimicrobial and antibiofilm activity of the essential oil from dried leaves of Eucalyptus staigeriana. Arquivos do Instituto Biológico 2019; 86. 63. Gitaari N, Kareru P, Githua M. Antimicrobial Potential of Pelargonium citrosum and Rosmarinus officinalis Essential Oils. Int Res J Pure Appl Chem. 2019: 1-5.