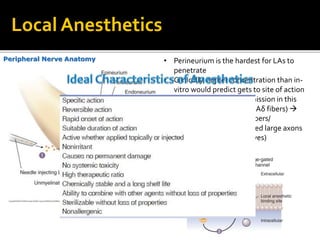
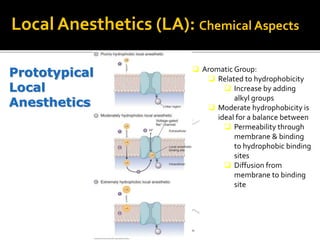
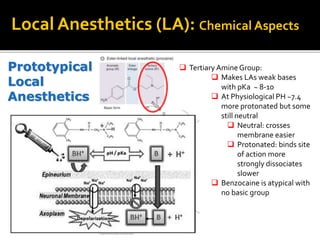
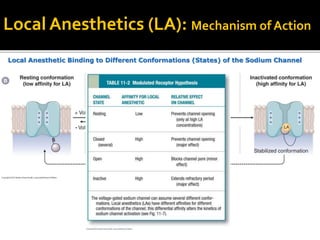
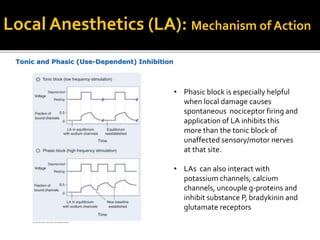
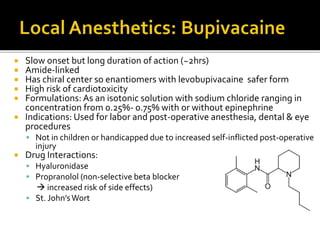
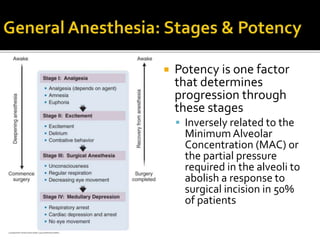
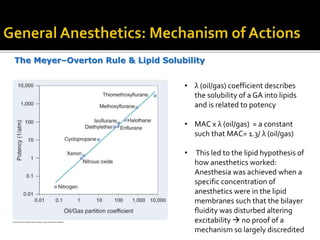
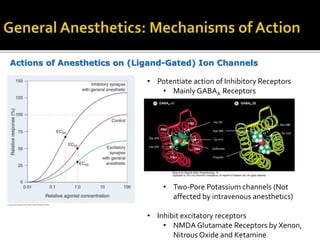
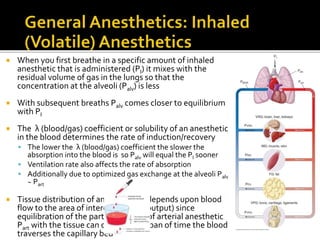
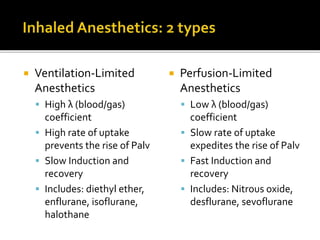
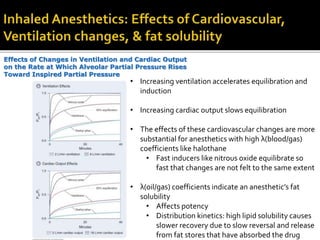
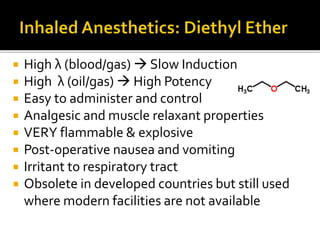
The document provides a comprehensive overview of local anesthetics, including their historical development, mechanisms of action, and various types such as topical, infiltration, peripheral nerve blocks, and central nerve blocks. It details the chemical structure, pharmacokinetics, and potential side effects or toxicity associated with different local anesthetics, also touching upon general anesthetics and their characteristics. Additionally, specific indications, drug interactions, and contraindications for various agents are discussed, emphasizing their clinical relevance in surgical and procedural settings.