New FDA Approved Drugs in Q1 2022.pdf
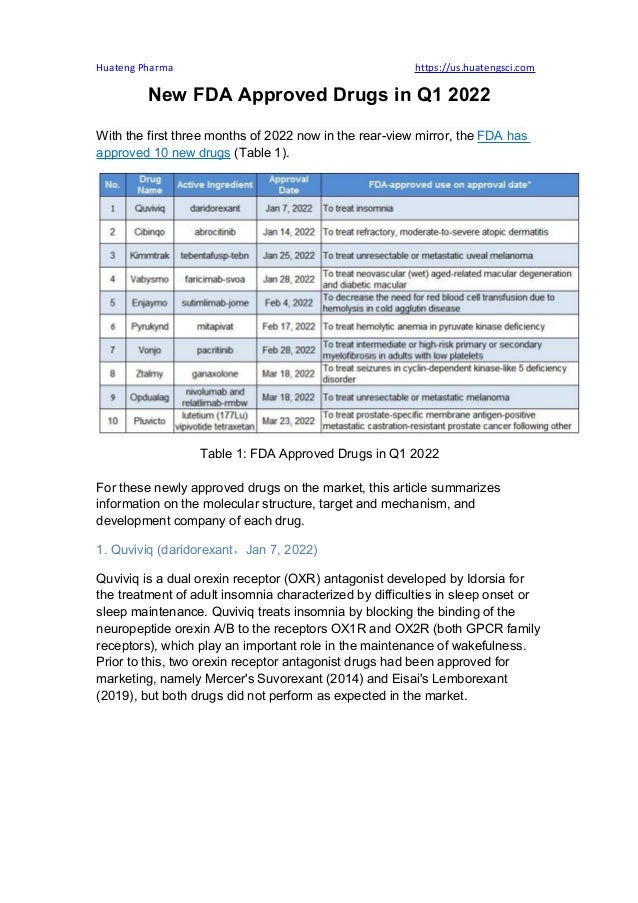
- 1. Huateng Pharma https://us.huatengsci.com New FDA Approved Drugs in Q1 2022 With the first three months of 2022 now in the rear-view mirror, the FDA has approved 10 new drugs (Table 1). Table 1: FDA Approved Drugs in Q1 2022 For these newly approved drugs on the market, this article summarizes information on the molecular structure, target and mechanism, and development company of each drug. 1. Quviviq (daridorexant,Jan 7, 2022) Quviviq is a dual orexin receptor (OXR) antagonist developed by Idorsia for the treatment of adult insomnia characterized by difficulties in sleep onset or sleep maintenance. Quviviq treats insomnia by blocking the binding of the neuropeptide orexin A/B to the receptors OX1R and OX2R (both GPCR family receptors), which play an important role in the maintenance of wakefulness. Prior to this, two orexin receptor antagonist drugs had been approved for marketing, namely Mercer's Suvorexant (2014) and Eisai's Lemborexant (2019), but both drugs did not perform as expected in the market.
- 2. Huateng Pharma https://us.huatengsci.com FDA approved orexin receptor antagonist drugs 2.Cibinqo (abrocitinib,Jan 14, 2022) Abrocitinib is a JAK1 inhibitor developed by Pfizer for the treatment of adult patients with refractory moderate-to-severe atopic dermatitis. Atopic dermatitis is one of the most common inflammatory skin diseases, with a prevalence of approximately 5-10% in the U.S. JAK is an intracellular protein kinase responsible for the downstream transmission of signal stimuli received by cytokine receptors or growth factor receptors on the cell membrane. First, JAK phosphorylates and activates STAT molecules, and the phosphorylated STAT translocates to the nucleus and regulates the transcriptional activation of target genes. The market for JAK inhibitors is attractive (estimated to be over $10 billion and growing) given the diversity and commonness of disease processes in which JAK is involved, and several JAK inhibitors are currently approved for marketing. JAK-STAT signaling pathway and inhibitors In 2011, Incyte/Novartis' JAK1/2 inhibitor Ruxolitinib was approved for the treatment of myelofibrosis. 2021, topical Ruxolitinib cream was approved for the treatment of vitiligo and mild to moderate atopic dermatitis, and is the only topical JAK inhibitor currently approved.
- 3. Huateng Pharma https://us.huatengsci.com In 2012, Pfizer's Tofacitinib was approved for the following indications: 1) moderate to severe rheumatoid arthritis (RA); 2) psoriatic arthritis (PsA); 3) moderate to severe ulcerative colitis ( Ulcerative Colitis (UC) and other autoimmune diseases. In 2017, Incyte/Lilly's JAK1/2 inhibitor Baricitinib was launched for the treatment of moderately severe active rheumatoid arthritis with an inadequate response to TNF antagonist therapy. Notably, Baricitinib was previously granted Emergency Use Authorization (EUA) by the FDA for the treatment of patients with COVID-19, and the new indication for Baricitinib on COVID-19 was discovered by AI pharmaceutical company BenevolentAI, sparking an instant buzz. In 2019, Abbvie's JAK1/2 inhibitor Upadacitinib was approved for the treatment of moderately severe active rheumatoid arthritis. 2019 then saw the development of Fedratinib, a JAK2 inhibitor, finally pushed to market by Celgene (now acquired by BMS) for the treatment of myelofibrosis. With the exception of BMS's Fedratinib, all other molecules have relatively similar structures. Despite the attractive prospects of JAK inhibitors, studies in recent years have found that JAK inhibitors increase the risk of severe heart disease, cancer and blood clots in patients, and the FDA requires related drugs to add black box warnings to their instructions. JAK inhibitors currently approved by the FDA 3. Kimmtrak (tebentafusp-tebn,Jan 25, 2022)
- 4. Huateng Pharma https://us.huatengsci.com Tebentafusp-tebn, a bispecific protein developed by Immunocore, consists of a soluble affinity-enhancing T cell receptor fused to an anti-CD3 effector that confers T cell recognition of gp100-positive tumor cells (gp100 is a glycoprotein highly expressed in melanocytes and melanoma) for the treatment of HLA-A *02:01-positive unresectable or metastatic adult uveal melanoma. Mechanism of action of tebentafusp-tebn (Figure source: N Engl J Med) Tebentafusp-tebn binds to uveal melanoma cells and activates polyclonal T cells to release inflammatory cytokines and cytolytic proteins that induce apoptosis in tumor cells. The median overall survival (mOS) of patients in the Tebentafusp-tebn treatment group was 21.7 months, and the median progression-free survival (mPFS) was 3.3 months. 4. Vabysmo (faricimab-svoa,Jan 28, 2022) Faricimab-svoa is a bispecific antibody developed by Genentech for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME). Faricimab-svoa blocks both angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A ( VEGF-A), which are thought to contribute to vision loss by destabilizing blood vessels, possibly further causing new leaky vessel formation and increasing inflammation. Preclinical studies suggest that inhibition of these two pathways has potential complementary benefits and may be effective in reducing vascular leakage and inflammation in the macula.
- 5. Huateng Pharma https://us.huatengsci.com Structure and target site of Faricimab-svoa 5. Enjaymo (sutimlimab-jome,Feb 4, 2022) Enjaymo, a lgG4 monoclonal antibody developed by Sanofi, is the "first-in-class" treatment for Cold Agglutinin Disease (CAD) to reduce the need for red blood cell transfusions due to hemolysis in adult patients with CAD. CAD is a rare form of autoimmune hemolytic anemia in which a patient's red blood cells are "tagged" with cold agglutinin, an antibody active at 3-4°C, which causes red blood cell clumping and lysis resulting in anemia. The annual incidence of CAD is about 1 in a million and most patients are 40-80 years old. Enjaymo inhibits hemolysis in CAD patients by inhibiting the classical complement pathway and specifically targeting the complement C1s protein (a serine protease), which prevents the deposition of complement regulators on the surface of red blood cells. Mechanism and efficacy of Enjaymo for CAD (Figure source: Blood, 2019)
- 6. Huateng Pharma https://us.huatengsci.com 6. Pyrukynd (mitapivat,Feb 17, 2022) Pyrukynd is the "first-in-class" pyruvate kinase (PK) allosteric activator developed by Agios for the treatment of hemolytic anemia caused by PK deficiency. Pyrukynd acts by binding to PK tetramers and increasing PK activity. There are four main PK isozymes in mammals, of which PK-R is expressed in erythrocytes. PK-R deficiency results in accumulation of the glycolytic intermediate 2,3-DPG, reduced ATP, shortened erythrocyte life span and chronic hemolysis. Mechanism of action of mitapivat 7. Vonjo (pacritinib,Feb 28, 2022) Pacritinib is an oral JAK2 kinase-selective inhibitor developed by CTI BioPharma for the treatment of adult patients with moderate or high-risk primary or secondary (post-geniculocytosis or post-primary thrombocythemia) myelofibrosis (MF). Pacritinib has activity against wild-type JAK2, mutant JAK2 (V617F) and FLT3, which contribute to the signaling of cytokines and growth factors associated with hematopoiesis and immunity. Myelofibrosis is usually associated with dysregulated JAK2 signaling. Pacritinib has higher inhibitory activity against JAK2 compared to its fellow families JAK1, JAK3 and TYK2. Pacritinib also exhibits inhibitory activity against other kinase families such as CSF1R and IRAK1, but its clinical relevance has yet to be investigated.
- 7. Huateng Pharma https://us.huatengsci.com Mechanism of action of Pacritinib 8. Ztalmy (ganaxolone,Mar 18, 2022) Ztalmy is a neuroactive steroidal gamma-aminobutyric acid (GABA) receptor positive modulator developed by Marinus Pharma for the treatment of seizures associated with cyclin-dependent kinase-like 5 (CDKL5) deficiency, a severe and rare genetic disorder characterized by early-onset, uncontrollable seizures and severe neurodevelopmental disorders. The disease is caused by mutations in the CDKL5 gene located on the X chromosome, which expresses a protein important for normal brain development and function. Patients in the Ztalmy-treated group had a 30.7% median reduction in 28-day major motor seizure frequency, compared with a 6.9% reduction in the placebo group, meeting the trial's primary endpoint.
- 8. Huateng Pharma https://us.huatengsci.com Classification of antiepileptic drugs (source: CNS Drugs, 2016) More than 20 antiepileptic drugs have been marketed, most of which reduce hyperexcitability by decreasing excitability or enhancing inhibitory neurotransmission. The targets involved include GABA aminotransferase (GABA-T), GABA transporter protein (GAT), synaptic vesicle protein 2A (SV2A), GABA receptors, NMDA receptors, AMPA receptors, and the voltage-gated potassium channel family (KCNQ, also known as the Kv7 family). Among them, Lacosamide, which was launched in 2008 (developed by the Belgium pharmaceutical company UCB) for the treatment of seizure epilepsy, is currently the top-selling antiepileptic drug (its sales in 2020 are $1.883 billion). 9. Opdualag (nivolumab and relatlimab-rmbw,Mar 18, 2022) Opdualag is a combination therapy of the BMS-developed PD-1 monoclonal antibody Nivolumab and the LAG-3 monoclonal antibody relatlimab for the treatment of patients aged 12 years and older with unresectable or metastatic melanoma.
- 9. Huateng Pharma https://us.huatengsci.com Mechanism of action and therapeutic effect of Opdualag The trial met its primary endpoint, progression-free survival (PFS), and Opdualag more than doubled the median PFS when compared to nivolumab monotherapy, 10.1 months (95% Confidence Interval [CI]: 6.4 to 15.7) versus 4.6 months. The Opdualag safety profile was similar to that previously reported for nivolumab.1,2 No new safety events were identified with the combination when compared to nivolumab monotherapy.1,2 Grade 3/4 drug-related adverse events were 18.9% in the Opdualag arm compared to 9.7% in the nivolumab arm.2 Drug-related adverse events leading to discontinuation were 14.6% in the Opdualag arm compared to 6.7% in the nivolumab arm. LAG-3 is another popular immune checkpoint target in addition to CTLA-4 and PD-1, and Relatlimab became the first LAG3-targeting antibody to be approved for marketing. 10. Pluvicto (lutetium (177Lu) vipivotide tetraxetan,Mar 23, 2022) Pluvicto is a "first-in-class" radiopharmaceutical developed by Novartis for the treatment of Metastatic Castration-Resistant Prostate Cancer (mCRPC), a prostate-specific membrane antigen (PSMA). Cancer (mCRPC). PSMA is a tumor-associated antigen and a type II transmembrane protein that is highly
- 10. Huateng Pharma https://us.huatengsci.com expressed in several malignant prostate cancer cells. Pluvicto consists of PSMA-617, a ligand that targets PSMA, coupled to the radioisotope lutetium 177Lu. Following intravenous administration of Pluvicto, PSMA-617 targets and binds to the surface of PSMA-expressing tumor cells and enters the cells during the receptor cycle, followed by the destruction of tumor cells by beta particles released by decay of 177Lu. Patients treated with Pluvicto + standard therapy had a 38% lower risk of death compared to patients treated with standard therapy only. Mechanism of action of Pluvicto. Image credit: ASCO 2021 Also, the FDA approved Locametz (Ga 68 gozetotide) as a radiological reagent for PET imaging of PSMA-positive lesions (requires intravenous injection). Previously, Ga 68 PSMA-11 (available in 2020) and Pylarify (available in 2021) were both approved by the FDA as diagnostic reagents for PET imaging of PSMA-positive lesions. Huateng Pharma is a one-stop contract development and manufacturing organization (CDMO) to supply researchers and companies with PEG derivatives and products used across the pharmaceutical value chain including intermediates, excipients, APIs, and reagents. References: . Paul Nathan et al. "Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma" Lancet 2021; 398: 803-816. Novel Drug Approvals for 2022, https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therape utic-biological-products/novel-drug-approvals-2022
- 11. Huateng Pharma https://us.huatengsci.com . https://rarediseases.org/rare-diseases/cold-agglutinin-disease/ . Novel Drug Approvals for 2022, https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therape utic-biological-products/novel-drug-approvals-2022 . https://ir.marinuspharma.com/news/news-details/2022/Marinus-Pharmaceuticals-Announ ces-FDA-Approval-of-ZTALMY-ganaxolone-for-CDKL5-Deficiency-Disorder/default.aspx . https://www.who.int/en/news-room/fact-sheets/detail/epilepsy . Wolfgang Löscher et al. CNS Drugs, 2016, 30, 1055–1077. . https://news.bms.com/news/corporate-financial/2022/U.S.-Food-and-Drug-Administration -Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlim ab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.a spx .