The Role of Four Lipid Components Of LNPs.pdf
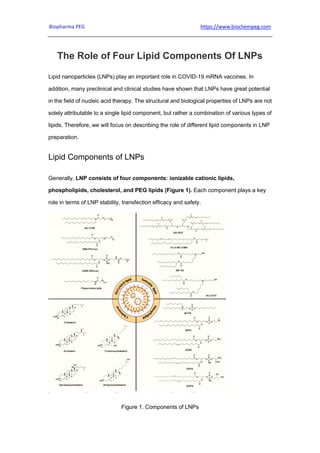
- 1. Biopharma PEG https://www.biochempeg.com The Role of Four Lipid Components Of LNPs Lipid nanoparticles (LNPs) play an important role in COVID-19 mRNA vaccines. In addition, many preclinical and clinical studies have shown that LNPs have great potential in the field of nucleic acid therapy. The structural and biological properties of LNPs are not solely attributable to a single lipid component, but rather a combination of various types of lipids. Therefore, we will focus on describing the role of different lipid components in LNP preparation. Lipid Components of LNPs Generally, LNP consists of four components: ionizable cationic lipids, phospholipids, cholesterol, and PEG lipids (Figure 1). Each component plays a key role in terms of LNP stability, transfection efficacy and safety. Figure 1. Components of LNPs
- 2. Biopharma PEG https://www.biochempeg.com Ionizable Cationic Lipids Ionizable cationic lipids are key components in the LNP formulation, and their acid dissociation constants (pKa) determine the ionization and surface charge of LNP, further affecting its stability and toxicity. Conventional permanently charged cationic lipids previously used for nucleic acid delivery (e.g., DOTAP) readily interact with negatively charged serum proteins and aggregate in the bloodstream, which can lead to rapid clearance of LNP by mononuclear phagocytes, resulting in a short half-life in the bloodstream. In addition, the relatively high hemolytic activity of cationic lipids increases the risk of toxic side effects, such as hemoglobin release due to red cell membrane damage. To avoid these problems, ionizable cationic lipids with pKa values typically ranging from 6.0 to 7.0 have been developed. This ionizable lipid-based LNP (iLNP) ensures efficient encapsulation of nucleic acids under acidic conditions and reduces toxicity during recycling under physiological conditions. Entering endosomes/lysosomes (pH below surface pKa), LNP can be positively charged again to facilitate endosome escape and release mRNA into the cytoplasm. It was found that LNP with pKa values of 6.2-6.5 and 6.6-6.9 favored hepatic delivery of siRNA in vivo and intramuscular administration of mRNA vaccines, respectively. Structurally, synthetic lipids usually contain three parts: (i) cationic or ionizable head groups, (ii) linker groups, and (iii) hydrophobic tails. The chemical diversity of each part results in a number of structurally distinct ionizable lipids that can be produced by combinatorial chemistry. Depending on the number of amino heads, ionizable cationic lipids can be classified as either monoamino or polyamino lipids. DLin-MC3-DMA (MC3), SM-102, and ALC-0315 are the best known monoamino acid lipids. They are also the three FDA-approved ionizable cationic lipids for RNA delivery. Researchers usually focus on tuning the lipid tail structure to confer enhanced potency or specific functions by changing the number of tails, designing linear or branching structures, and introducing unsaturated or biodegradable bonds, e.g., the unsaturated tails in MC3 and ester bonds in L319 play important roles in facilitating endosomal escape of siRNAs and accelerating intracellular degradation of lipids. By providing larger head groups, polyamino-ionizable cationic lipids have greater variability than monoamino-ionizable cationic lipids, and a
- 3. Biopharma PEG https://www.biochempeg.com number of multitude of lipids have been designed and studied, e.g., 306O i10, cKK-E12, C12-200, 5A2-SC8, TT3, FTT5. Figure 2. The ionizable lipids used Comirnaty® (ALC-0315) and Spikevax® (SM-102) and in Onpattro® (MC3). Source: reference [1] PEG-lipids Even though PEG-lipids constitute the smallest molar percentage of the lipid components in LNPs (typically ∼1.5 mol%), they have several effects on the properties of lipid nanoparticles. 1. The amount of PEG-lipids can influence particle size and zeta potential; 2. PEG-lipids can better contribute to particle stability by decreasing particle aggregation; 3. Certain PEG modifications extend the blood circulation time of nanoparticles by lowering clearance mediated by the kidneys and the mononuclear phagocyte system (MPS). 4. PEG-lipids can be used to conjugate particular ligands to the particle for targeted delivery. The short chained diacyl PEG-lipid PEG-carbamate-1,2-dimyristoyl-sn-glycerol (PEG-c-DMG) is introduced in the Onpattro® formulation, which is rapidly dissociated from the lipid membrane. When dissociated from their PEG, LNPs can interact with blood proteins, including ApoE proteins, for delivery to the hepatocytes. In this context, two short lipids-PEG (C14) were recently used in the Comirnaty® and Spikevax® COVID-19 vaccines: ALC-0159 (DTA-PEG2000, ditetradecylacetamide) and DMG-PEG2000, respectively.
- 4. Biopharma PEG https://www.biochempeg.com Figure 3. The PEG-lipids used in Onpattro® (PEG-c-DMG) , Spikevax® (PEG-DMG) and Comirnaty® (ALC-0159). Source: reference [1] However, PEGylation also presents a "PEG dilemma" that hinders interaction with target cells and subsequent endosomal escape, resulting in reduced transfection efficiency. Accelerated blood clearance ("ABC phenomenon") is an unexpected immunogenic response observed in PEG conjugates that results in rapid clearance of PEGylated nanocarriers. The ABC phenomenon has been widely observed after repeated dosing and reduces the effectiveness of PEG conjugates and nanocarriers. Another unexpected immune response is the hypersensitivity reaction known as complement activation-related pseudoallergy (CARPA), which significantly reduces the safety of PEGylated nanocarriers and has been associated with reduced efficacy of PEGylated treatments in clinical trials. The CARPA phenomenon has been categorized as a non-IgE-mediated pseudoallergic reaction caused by activation of the complement system. Various parameters of the PEG–lipid chemical structure including PEG size and architecture, lipid structure including hydrocarbon chains, headgroup, lipid–PEG linkage, and PEG terminal groups, are significant determinants of the PEGylated LNPs safety and bioactivity.
- 5. Biopharma PEG https://www.biochempeg.com Figure 3. Scheme of the PEG–lipid structure. Source: reference [3] ▶ PEG length is a key structural factor influencing the immunologic safety profile. The effect is biphasic, with both long and short chain PEG conjugates being more likely to induce the ABC phenomenon. ▶ Like PEG length, PEG density (i.e., the percentage of PEG in the LNP) also exhibits a biphasic effect. However, both lower and higher densities of PEG exhibit a reduced ABC phenomenon. ▶ Differences in PEG structure may have an effect, with branched PEG lipid conjugates conferring a higher degree of invisibility to LNP compared to linear PEG. ▶ The functional terminal groups attached to the chain of the PEG particles are another factor that affects their immunogenicity and clearance. ▶ Parameters such as size and surface charge also affect immunogenicity. For example, PEGylated carriers containing negatively charged phospholipids are more capable of stimulating the immune system through complement activation than uncharged vesicles. ▶ As with the PEG fraction, the structure and length of the hydrophobic chains of lipids can determine not only the degree of immunogenicity but also its efficacy. ▶ PEGylated LNP produced using a specific lipid anchoring group (e.g., cholesterol as an anchoring group) has more sustained permeability and higher systemic bioavailability in circulation. ▶ Lipid linkage is an important parameter in the design and performance of lipids, where ester bonds replace carbamate bonds for the formation of unstable vesicles.
- 6. Biopharma PEG https://www.biochempeg.com A thorough understanding of the correlation between the structural parameters of PEG lipids and the immune-related adverse effects of PEGylated LNPs, as well as their biophysical and physiological background, is an essential requirement. With this knowledge, optimal drug formulations that reduce and eliminate adverse immune reactions and improve the safety and efficiency of PEGylated drugs can be prepared. Helper Lipids - Cholesterol The inclusion of cholesterol in nucleic acid-containing LNP formulations is based primarily on two major findings obtained with liposomal formulations of small molecule therapeutics: 1) cholesterol is an exchangeable molecule that can accumulate within liposomes during circulation, 2) cholesterol dramatically reduces the amount of surface-bound proteins and improves the circulating half-life. Therefore, equimolar amounts of cholesterol are included in LNP formulations relative to endogenous membranes; this prevents net efflux or influx and maintains membrane integrity. Lipids such as cholesterol are also essential for encapsulating nucleic acids. Since cholesterol increases membrane rigidity, it reduces drug leakage from the liposomal core. However, for large cargo such as nucleic acids, this effect may not be important. As the design of ionizable lipids has improved, providing more potency and less toxicity, the total proportion of helper lipid has decreased. However, a recent study noted that a certain threshold amount of helper lipids is still needed to promote stable encapsulation. Biopharma PEG can supply high-quality cholesterol (plant-derived) from lab to large scale to meet your research and drug development needs.
- 7. Biopharma PEG https://www.biochempeg.com Figure 4. The molecular shape of standard helper lipids, including DSPC, DOPE, and cholesterol, used in LNPs. Source: reference [1] Helper Lipids – Phospholipids Phospholipids are helper lipids that contribute to the formation of lipid nanoparticles and the escape of endosomes. Commonly used phospholipids in preclinical studies and clinical applications are DSPC and DOPE. Phospholipids can spontaneously organize into lipid bilayers and the higher phase transition temperature enhances the membrane stability of LNPs. Similar to cell membranes, phospholipids are located at the periphery of the LNP. These lipids are usually semisynthetic. For example, phosphatidylcholine is usually derived from natural sources such as egg yolk and soybeans and can be chemically modified (e.g., by adding fatty acid tails). DSPC is a structural lipid used in LNPs for siRNA therapy (Onpattro) and SARS-CoV-2 mRNA vaccines. DSPC consists of a phosphatidylcholine head group and two saturated 18-carbon tails, with the two tails forming a tightly packed lipid bilayer. In LNP, DSPC is mainly located on the surface of the nanoparticles and in more marginal positions in the core of the nanoparticles. DOPE is another phospholipid commonly used in preclinical studies of LNP. The unsaturated tails of DOPE not only form a more fluid lipid bilayer but also are capable of forming a hexagonal-phase (HII) organized form, which facilitates the fusion of lipid membranes with endosomal membranes, leading to cytoplasmic nucleic acid release.
- 8. Biopharma PEG https://www.biochempeg.com Conclusion Under the premise of consistent preparation conditions, the nucleic acid composition and the proportion of lipids have a decisive influence on the structure of nanoparticles. Understanding the function of various types of lipids is a prerequisite for the optimization of LNP formulations, and the roles of four common components of LNPs are briefly described in the paper. Biopharma PEG is a dynamic science company dedicated to PEG derivatives. We are capable of supplying small to large quantities of a rich selection of PEG derivatives with GMP & non-GMP standards for your mRNA vaccine R&D. We can produce and provide classic PEG lipids, such as mPEG-DMG, ALC-0159 and mPEG-DSPE for your LNPs R&D. References: [1] Hald Albertsen C, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev. 2022 Sep;188:114416. doi: 10.1016/j.addr.2022.114416. Epub 2022 Jul 3. PMID: 35787388; PMCID: PMC9250827. [2] Hou, X., Zaks, T., Langer, R. et al. Lipid nanoparticles for mRNA delivery. Nat Rev Mater 6, 1078–1094 (2021). https://doi.org/10.1038/s41578-021-00358-0 [3] PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective, Rumiana Tenchov, Janet M. Sasso, and Qiongqiong Angela Zhou, Bioconjugate Chemistry 2023 34 (6), 941-960, DOI: 10.1021/acs.bioconjchem.3c00174 Related Articles: Application of Lipid Nanoparticles In Vaccine And Drug Delivery Liposomes vs. Lipid Nanoparticles: Which Is Best for Drug Delivery? Lipid nanoparticles (LNPs) In Cancer Immunotherapy PEGylated Liposomes in the Clinic and Clinical Trials