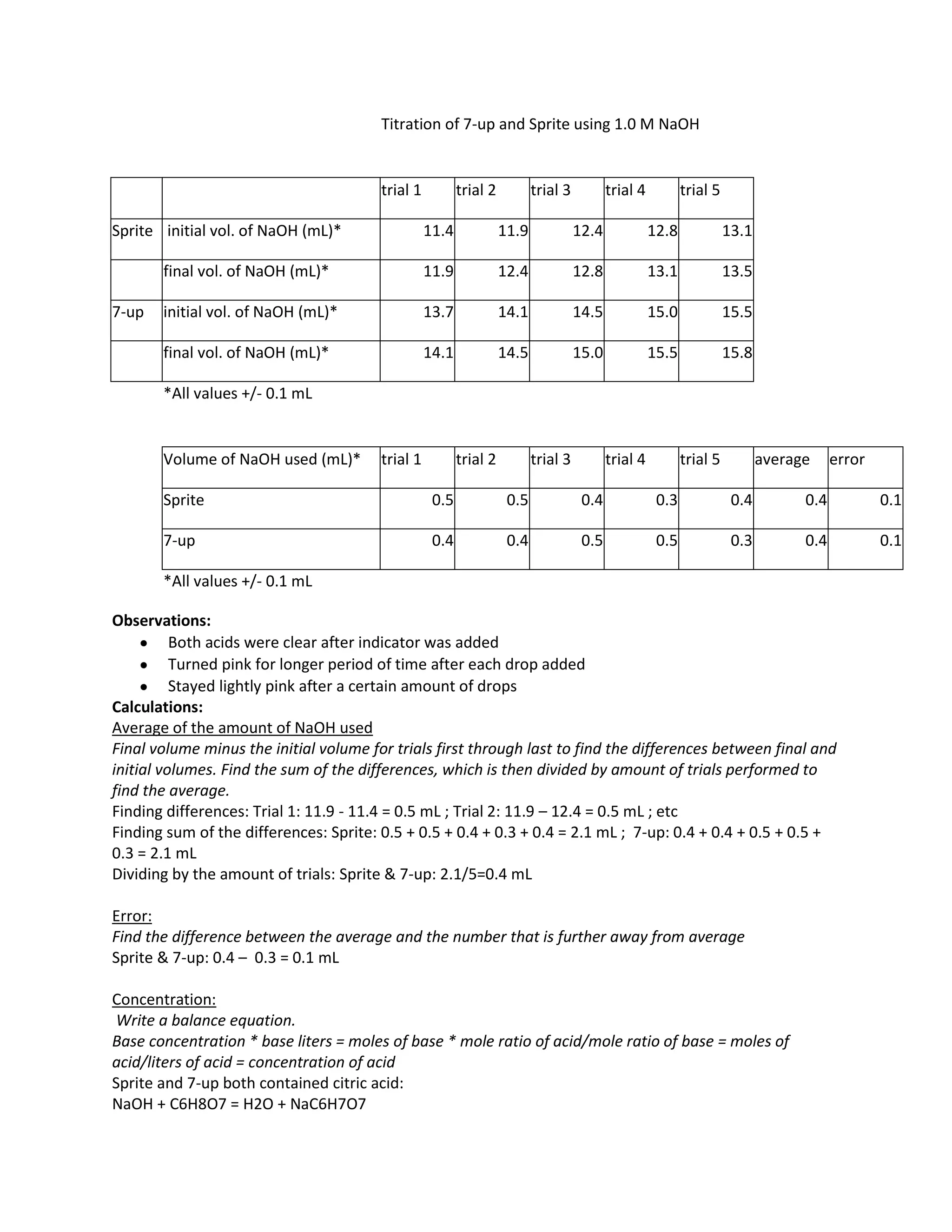
The document summarizes an experiment titrating 7-Up and Sprite with 1.0 M NaOH to determine the concentration of citric acid in each drink. The average volume of NaOH needed to neutralize the citric acid was 0.4 mL for both drinks, indicating they have the same concentration of 0.04 M citric acid. The error in the experiment was 0.1 mL due to inaccurate measurements and overshooting during titration. More precise equipment and using different acids could improve the experiment.