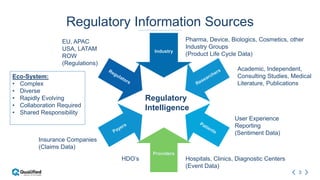
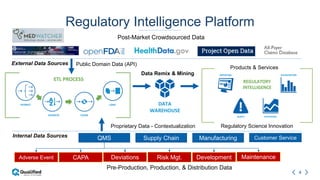
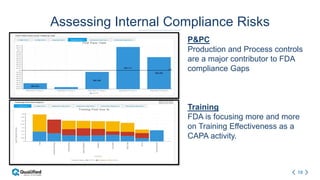
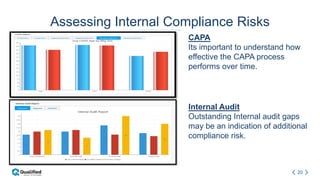
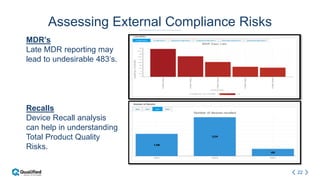
Regulatory intelligence involves acquiring knowledge through analyzing various internal and external information sources to enable timely, data-driven decision making in the complex and evolving global regulatory landscape. A regulatory intelligence platform can integrate internal quality and compliance data with external sources like FDA reports, clinical studies, and regulations. This provides a holistic view to identify risks, prioritize improvements, and help maintain market advantage. However, many organizations still struggle with data access, analysis, and using analytics to impact business outcomes. Assessing internal audit and complaint data alongside external benchmarks can help evaluate inspection readiness and prioritize compliance issues.