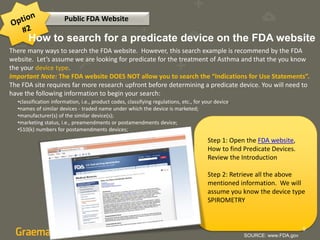
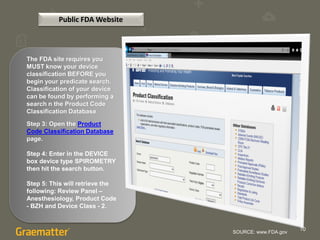
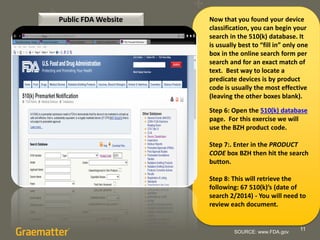
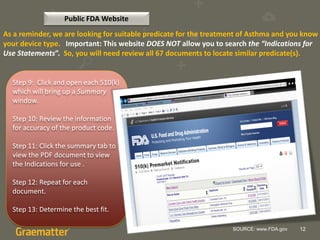
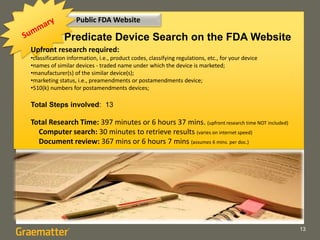
The document compares two methods for locating predicate devices for 510(k) submissions: using Graematter's Sofie regulatory intelligence database and the public FDA website. The Sofie system allows for a quicker search with a total research time of 45 minutes, while the FDA website requires extensive upfront research resulting in a total time of 397 minutes. Each method utilizes the same government data but offers different search capabilities and user experiences.













![SOURCE: www.FDA.gov 20
What’s a 510(k)?
The FDA’s A Premarket Notification [510(k)] is a premarketing submission made
to FDA to demonstrate that the device to be marketed is safe and effective by
proving substantial equivalence1 (SE) to a legally marketed device (predicate
device) that is not subject to Premarket Approval (PMA). Submitters must
compare their 510(k) device to a similar legally marketed U.S. device(s). A
device recently cleared under 510(k) is usually used as a predicate device.
However, any legally U.S. marketed device may be used as a predicate. This
includes: a device that has been cleared through the 510(k) process; a device
that was legally marketed prior to May 28, 1976 (preamendments device); a
device that was originally on the U.S. market as a Class III device (Premarket
Approval) and later downclassified to Class II or I; or a 510(k) exempt device.](https://image.slidesharecdn.com/predicate-140326104433-phpapp01/85/Regulatory-Intelligence-Series-How-to-find-Predicate-Devices-SOFIE-compared-to-FDA-Site-20-320.jpg)
