Quantum Number.pdf
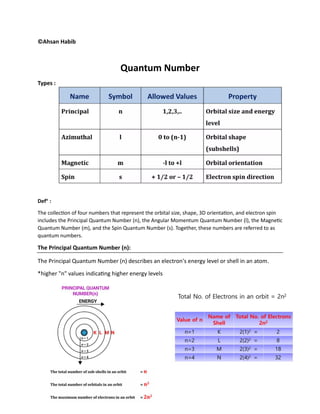
- 1. ©Ahsan Habib Quantum Number Types : Defn : The collection of four numbers that represent the orbital size, shape, 3D orientation, and electron spin includes the Principal Quantum Number (n), the Angular Momentum Quantum Number (l), the Magnetic Quantum Number (m), and the Spin Quantum Number (s). Together, these numbers are referred to as quantum numbers. The Principal Quantum Number (n): The Principal Quantum Number (n) describes an electron's energy level or shell in an atom. *higher "n" values indicating higher energy levels
- 2. Azimuthal Quantum number : This number defines the shape or subshell of an electron's orbital within a particular energy level. Magnetic Quantum Number, m This number specifies the orientation of an orbital within a subshell.
- 3. 4.Spin Quantum Number , s Each orbital accommodate two electrons with opposite spins. Question: calculate the total number of electrons in the n=3 Energy level l Sub shell/sub level m Number of orbital,(2l+1) Number of electrons (Each orbital accommodate two electrons with opposite spins.) n=3 0 3s 0 1 2 1 3p -1, 0, 1 3 6 2 3d -2, -1, 0, 1, 2 5 10 total number of e= 18
- 4. Homework: calculate the total number of electrons in the n=4 Which quantum number specifies the orientation of an orbital in 3D space? a) Principal Quantum Number (n) b) Azimuthal Quantum Number (l) c) Magnetic Quantum Number (m) d) Spin Quantum Number (s) In an atom with n = 5, how many total electrons can be accommodated in all the orbitals of this energy level? a) 10 b) 14 c) 18 d) 32 How many p orbitals are there in the n = 3 energy level? a) 1 b) 2 c) 3 d) 6 What is the maximum number of electrons that can occupy a single orbital in an atom? a) 1 b) 2 c) 4 d) 8 If the Principal Quantum Number (n) is 4, how many possible values of the Azimuthal Quantum Number (l) are there? a) 1 b) 2 c) 3 d) 4 An electron with a magnetic quantum number (m) of +2 belongs to which type of orbital? a) s b) p c) d d) f How many different magnetic quantum numbers (m) are possible for an electron in a p subshell? a) 1 b) 2 c) 3
- 5. d) 4 In an atom with n = 6, how many different subshells are there? a) 1 b) 2 c) 3 d) 4 Which quantum number describes the energy level or shell of an electron? a) Principal Quantum Number (n) b) Azimuthal Quantum Number (l) c) Magnetic Quantum Number (m) d) Spin Quantum Number (m) How many electrons can fill a single d subshell completely? a) 2 b) 4 c) 6 d) 10 What is the maximum number of electrons that can be accommodated in an energy level with Principal Quantum Number (n) equal to 7? a) 7 b) 14 c) 28 d) 56 The Spin Quantum Number (s) represents: a) The orientation of the orbital b) The direction of electron motion c) The electron's energy level d) The direction of electron spin In an atom with n = 5, how many orbitals are there in the p subshell? a) 1 b) 3 c) 5d) 7 What is the maximum number of electrons that can be accommodated in a single f subshell? a) 2 b) 4 c) 6 d) 14
- 6. For the magnetic quantum number (m) of -1, which type of orbital does it correspond to? a) s b) p c) d d) f Soln : b) 14 c) 3 b) 2 d) 4 b) p b) 2 d) 4 a) Principal Quantum Number (n) d) 10 c) 28 d) The direction of electron spin b) 3 d) 14 b) p