properties-of-water ppt
•Download as PPT, PDF•
0 likes•3 views
READY TO DOWNLOAD
Report
Share
Report
Share
Recommended
Recommended
More Related Content
Similar to properties-of-water ppt
Similar to properties-of-water ppt (20)
Intermolecular_Forces_Liquids_and_Solids-Autosaved.ppt

Intermolecular_Forces_Liquids_and_Solids-Autosaved.ppt
General Chemistry 2_IMF and Properties of Liquids.pptx

General Chemistry 2_IMF and Properties of Liquids.pptx
Experimental Questions 1- Which types of compounds have the strongest.docx

Experimental Questions 1- Which types of compounds have the strongest.docx
Chapter 11 Lecture- Intermolecular Forces, Liquids, & Solids

Chapter 11 Lecture- Intermolecular Forces, Liquids, & Solids
Recently uploaded
Recently uploaded (20)
THE ROLE OF BIOTECHNOLOGY IN THE ECONOMIC UPLIFT.pptx

THE ROLE OF BIOTECHNOLOGY IN THE ECONOMIC UPLIFT.pptx
FAIRSpectra - Enabling the FAIRification of Spectroscopy and Spectrometry

FAIRSpectra - Enabling the FAIRification of Spectroscopy and Spectrometry
Bhiwandi Bhiwandi ❤CALL GIRL 7870993772 ❤CALL GIRLS ESCORT SERVICE In Bhiwan...

Bhiwandi Bhiwandi ❤CALL GIRL 7870993772 ❤CALL GIRLS ESCORT SERVICE In Bhiwan...
PATNA CALL GIRLS 8617370543 LOW PRICE ESCORT SERVICE

PATNA CALL GIRLS 8617370543 LOW PRICE ESCORT SERVICE
GBSN - Biochemistry (Unit 2) Basic concept of organic chemistry 

GBSN - Biochemistry (Unit 2) Basic concept of organic chemistry
Use of mutants in understanding seedling development.pptx

Use of mutants in understanding seedling development.pptx
Human & Veterinary Respiratory Physilogy_DR.E.Muralinath_Associate Professor....

Human & Veterinary Respiratory Physilogy_DR.E.Muralinath_Associate Professor....
Genetics and epigenetics of ADHD and comorbid conditions

Genetics and epigenetics of ADHD and comorbid conditions
TransientOffsetin14CAftertheCarringtonEventRecordedbyPolarTreeRings

TransientOffsetin14CAftertheCarringtonEventRecordedbyPolarTreeRings
Call Girls Ahmedabad +917728919243 call me Independent Escort Service

Call Girls Ahmedabad +917728919243 call me Independent Escort Service
Role of AI in seed science Predictive modelling and Beyond.pptx

Role of AI in seed science Predictive modelling and Beyond.pptx
Climate Change Impacts on Terrestrial and Aquatic Ecosystems.pptx

Climate Change Impacts on Terrestrial and Aquatic Ecosystems.pptx
Cyathodium bryophyte: morphology, anatomy, reproduction etc.

Cyathodium bryophyte: morphology, anatomy, reproduction etc.
300003-World Science Day For Peace And Development.pptx

300003-World Science Day For Peace And Development.pptx
properties-of-water ppt
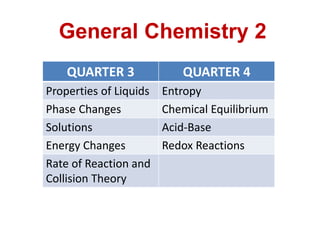
- 1. QUARTER 3 QUARTER 4 Properties of Liquids Entropy Phase Changes Chemical Equilibrium Solutions Acid-Base Energy Changes Redox Reactions Rate of Reaction and Collision Theory General Chemistry 2
- 2. Intermolecular Forces 11.2 Intermolecular forces are attractive forces between molecules. Intramolecular forces hold atoms together in a molecule.
- 3. Properties of Liquids and Solids
- 4. “Does high temperature affect the survival of the coronavirus ?”
- 5. “Does high temperature affect the survival of the coronavirus ?” • Areas with low temperature could increase the rate of spread of related diseases caused by coronavirus compared to tropical countries • It can be inferred that the activity of the virus is affected by temperatre.
- 6. • Likewise, solids and liquids are affected by temperature. The physical properties of liquids and solids such as temperature is greatly due to the intermolecular forces present between molecules. Other properties that are also affected by it are • surface tension • Viscosity • vapor pressure • and molar heat of vaporization
- 7. Effect of Intermolecular forces to some Physical Properties 1. Surface tension is the amount of energy required to stretch or increase the surface of a liquid by a unit area. Strong intermolecular forces High surface tension 11.3 The molecules at the surface of a liquid are pulled in all directions. Directions like downward and sideways not upward or away from the surface, however if the hydrogen bonds are disrupted, the surface tension will decrease.
- 8. The force of the surface tension of the water balances the basilisk lizard`s weight helping it to walk on water.
- 9. Cohesion is the intermolecular attraction between like molecules 11.3 Adhesion is an attraction between unlike molecules Adhesion Cohesion attracted to glass attracted to each other 2. Capillary Action
- 10. Figure 1a shows the water molecules are attracted to other molecules which is the molecules of the beaker figure 1b is more on adhesion wherein the Hg molecules did not get attracted to the walls of the beaker.
- 11. 3. Viscosity is a measure of a fluid’s resistance to flow. 11.3 Strong intermolecular forces High viscosity
- 12. Which of these compounds has the highest intermolecular forces? Which is more viscous hexane and decane?
- 13. Which of these compounds has the highest intermolecular forces? Glycerol -more build-up hydrogen bond. Higher IMF the more viscous it is. Another point to consider is the size of the molecule, a liquid that has a long chain of hydrocarbon has the greater intermolecular attraction. Which is more viscous hexane and decane?
- 14. 4. Vapour Pressure Which figure below do you think will have more molecules to turn into gaseous state at STP condition?
- 15. 4. Vapour Pressure Which figure below do you think will have more molecules to turn into gaseous state at STP condition? acetone - its molecules easily escape from liquid to gases and if this happens this would mean that the interaction between molecules of acetone is weak. Since it is a closed jar, the amount of escaped gas molecules will now create a particular amount of pressure. The pressure that is created by these bouncing molecules of acetone is called vapor pressure.
- 16. 5. Molar Heat of Vaporization ( Hvap) When we say boiling point, it is the temperature at which the liquid converts into gas. Meaning it is the temperature where the vapor pressure of a liquid equals the external pressure (at equilibrium point). This explains why water boils or why liquid boils. Now, for the water molecule to vaporize 1 mole of a liquid at 100 degrees Celsius, this requires an energy which is called molar heat of vaporization. boiling point increases the amount of energy required to vaporize also increases.
- 17. Remember: Matter escapes into different phases their properties also change and these properties are influenced by the way they get attracted to one another.