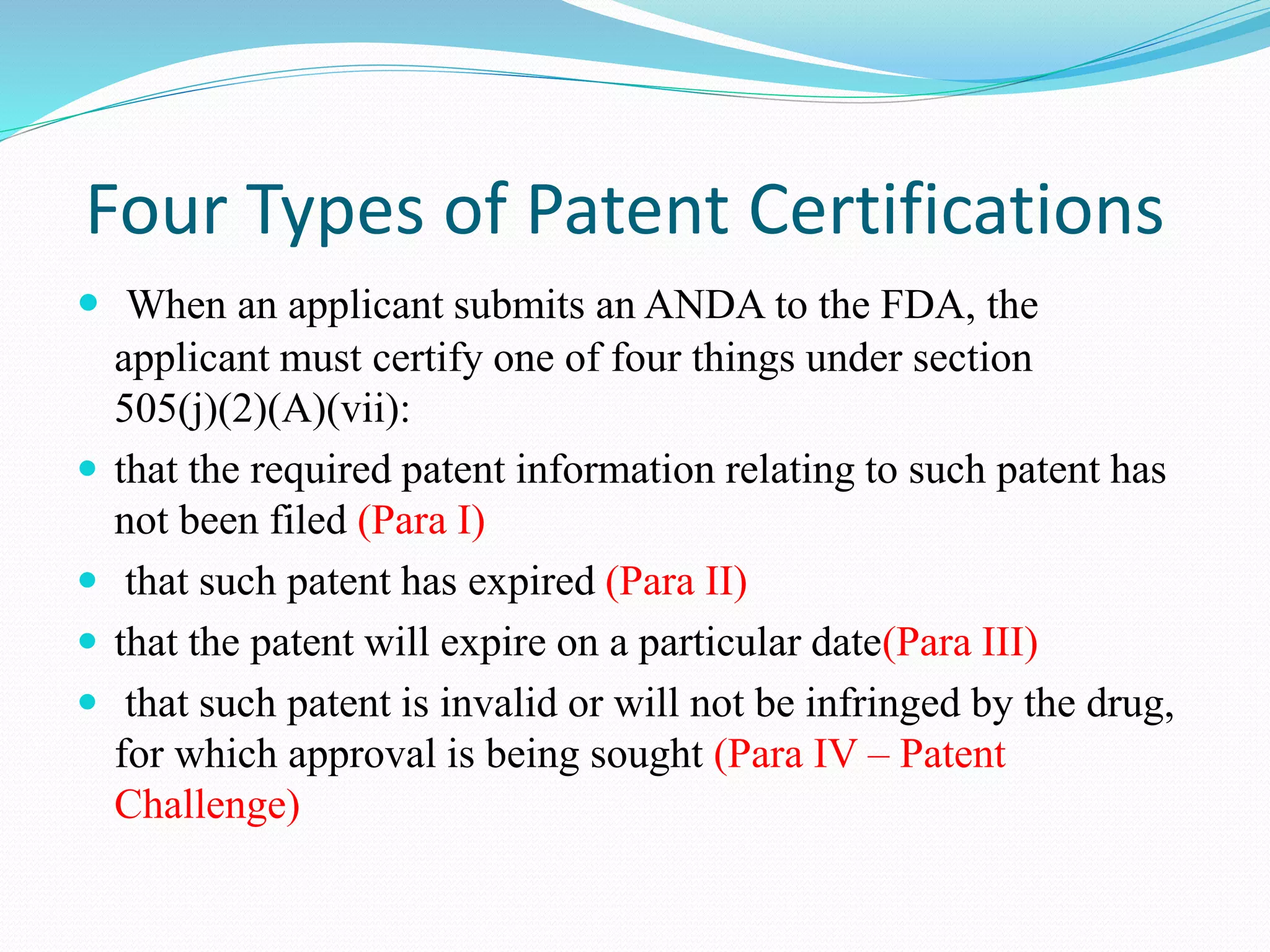
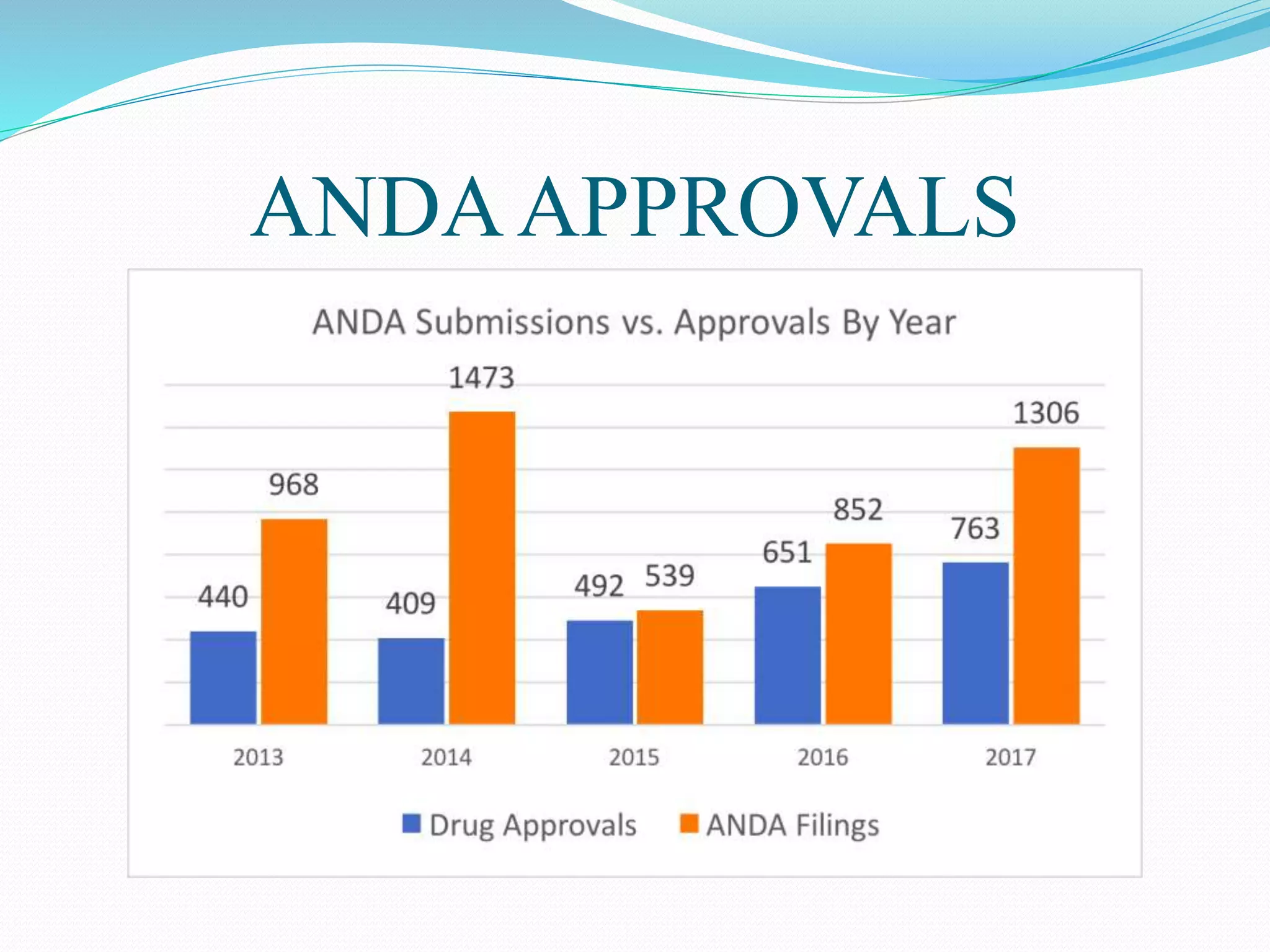
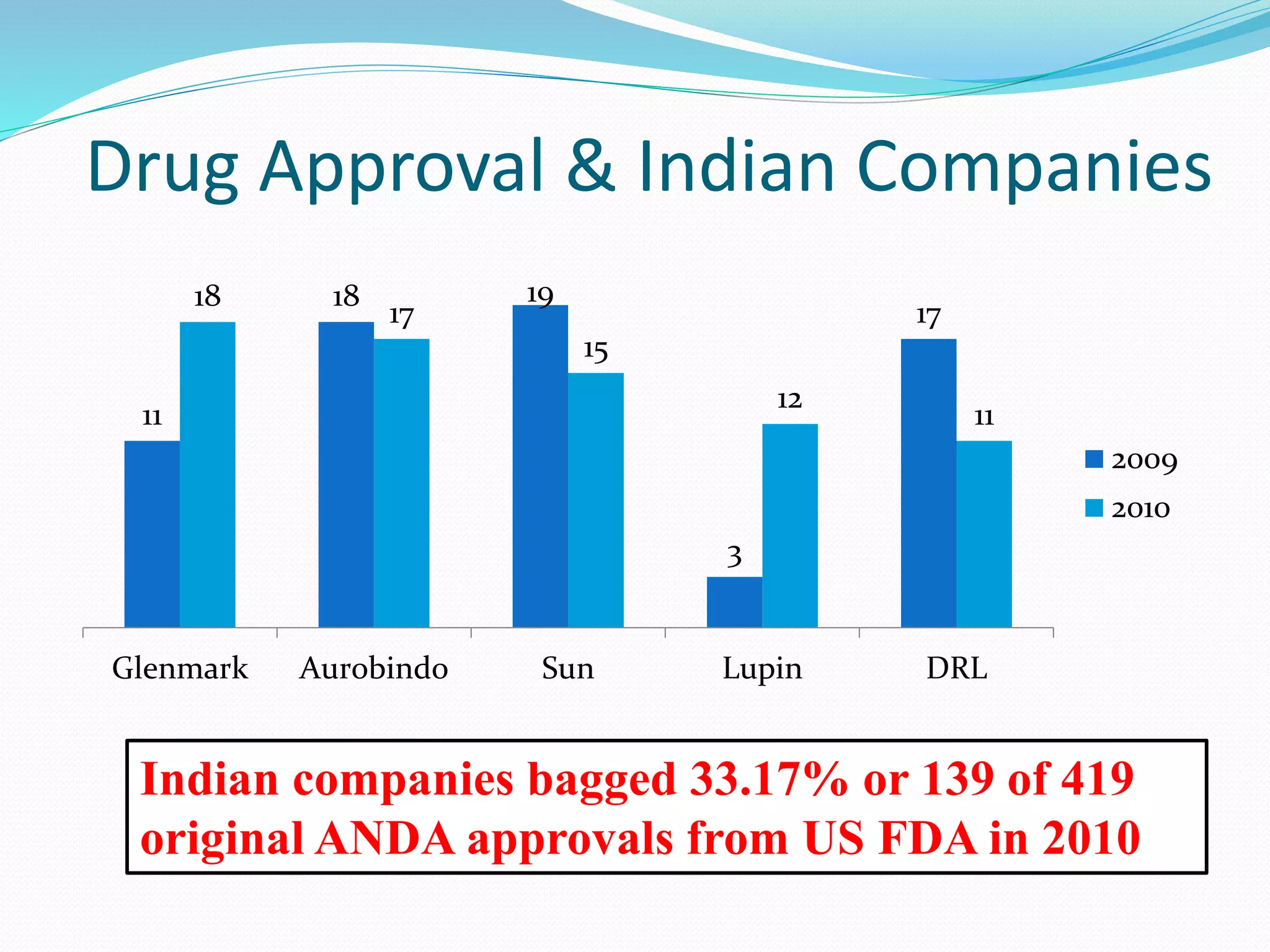
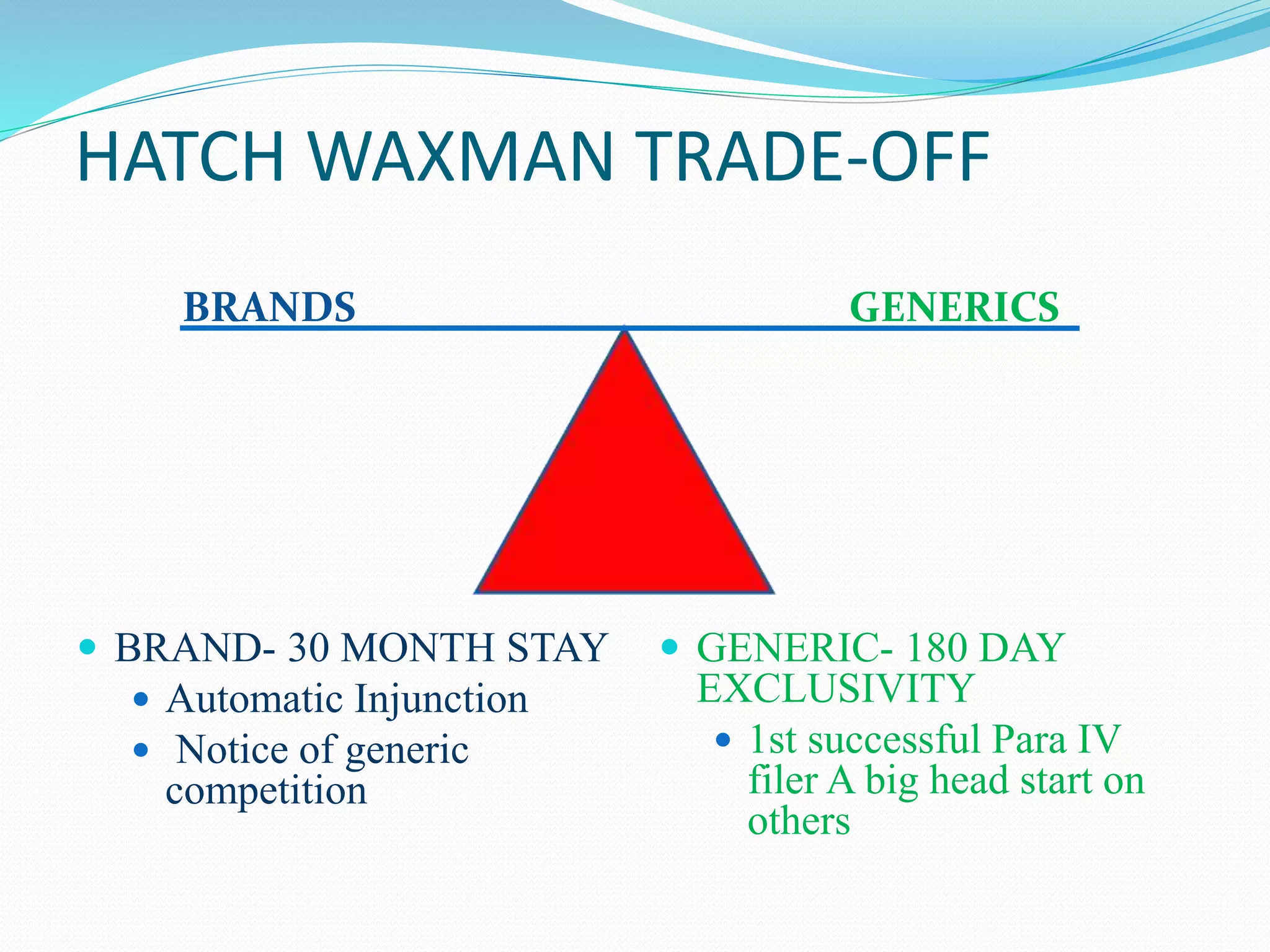
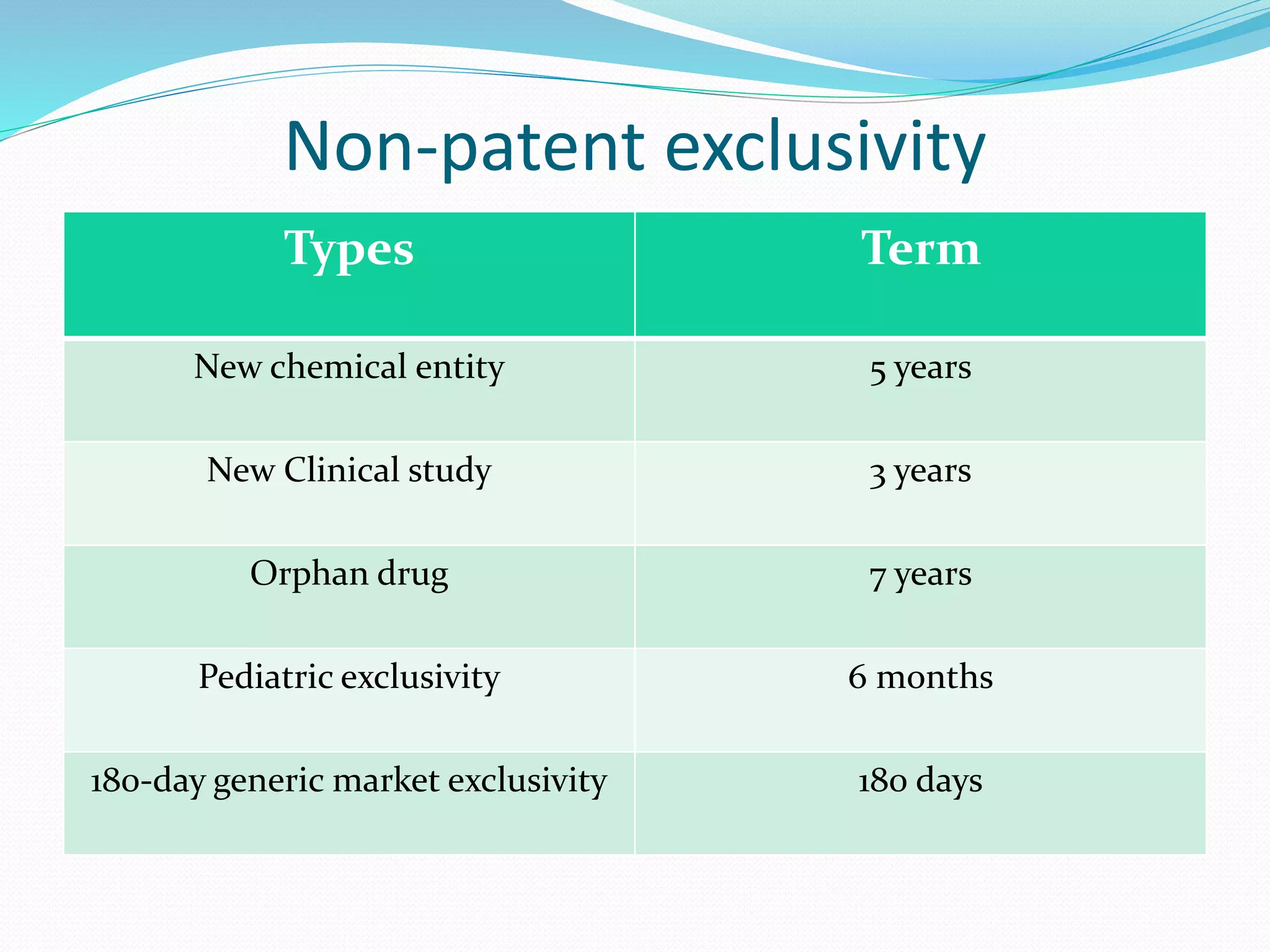
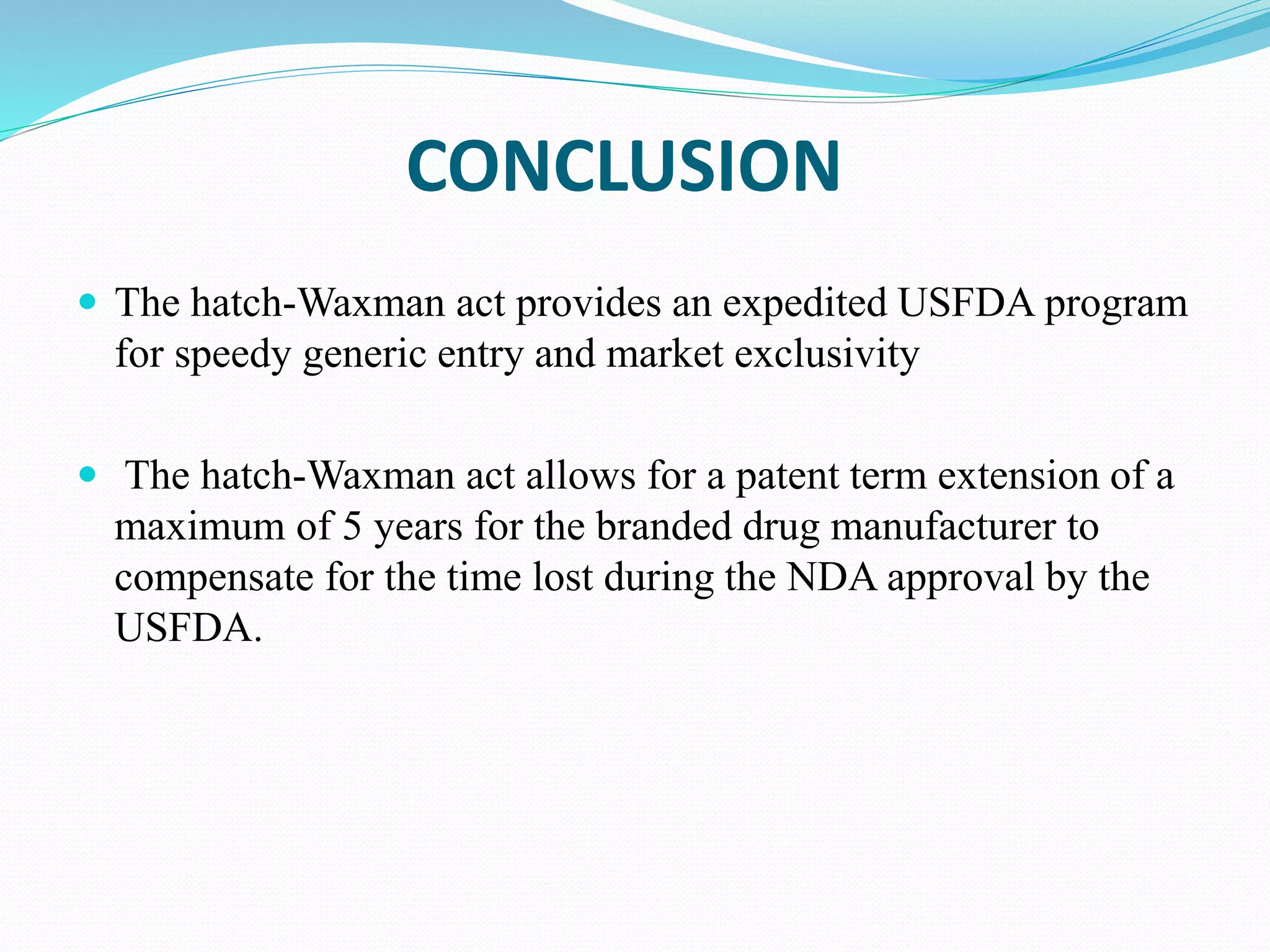
This document summarizes the Hatch-Waxman Act, which established the modern system for regulating generic drugs in the US. The Act aims to make generic drugs more accessible by streamlining the approval process for generics while also providing incentives to brand name drug companies. It allows generics to challenge drug patents and grants exclusivity periods to first generic applicants and brand name drugs for new chemical entities. The Orange Book lists approved drug products and related patent information.
![Presented by
Mr. AKSHAY PATIL
M pharm 1st yr
[Pharmaceutics dept]
Guided by
Dr. A.J.Shinde Sir
ASSOCIATE PROFESSOR
M Pharm , Ph.D
DEPARTMENT OF PHARMACEUTICS](https://image.slidesharecdn.com/akshayseminar-191013170849/75/hatch-waxman-act-amendments-1-2048.jpg)