IB Chemistry on Redox Titration, Biological Oxygen Demand and Redox.
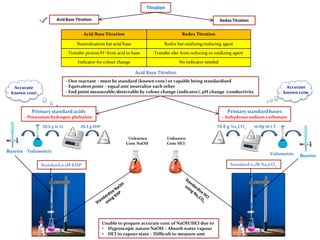
- 1. Titration Redox TitrationAcid Base Titration Primary standard acids - Potassium hydrogen phthalate Primary standard bases - Anhydrous sodium carbonate 10.6 g Na2CO3 Standard 0.1M Na2CO3 10.6g in 1 L Volumetric Burette Accurate known conc Unable to prepare accurate conc of NaOH/HCI due to • Hygroscopic nature NaOH – Absorb water vapour • HCI in vapour state – Difficult to measure amt VolumetricBurette Standard 0.1M KHP 20.4 g KHP20.4 g in 1L Unknown Conc NaOH Unknown Conc HCI ? ? Accurate known conc Acid/Base Titration Redox Titration Neutralization bet acid/base Redox bet oxidizing/reducing agent Transfer proton/H+ from acid to base Transfer elec from reducing to oxidizing agent Indicator for colour change No indicator needed Acid Base Titration - One reactant – must be standard (known conc) or capable being standardised - Equivalentpoint – equal amt neutralize each other - End point measurable/detectable by colour change (indicator), pH change /conductivity
- 2. Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2- KIO3 Vitamin C OCI-/Cu2+ Oxalate/ C2O4 2- Titration Redox TitrationAcid Base Titration Burette/Titrant Oxidizing agent ? Acid/Base Titration Redox Titration Neutralization bet acid/base Redox bet oxidizing/reducing agent Transfer proton/H+ from acid to base Transfer elec from reducing to oxidizing agent Indicator for colour change No indicator needed Redox Titration - One reactant – must be standard (known conc) or capable being standardised - Reaction bet Oxidizing agent/Titrant with Reducing agent/Analyte - Titrant of known concentration - Stoichiometrically equivalentamt titrant/titrand added - No indicator needed. Detectable by colour change of Oxidizing/Reducing agent Analyte/reducing agent Titrand Redox Titration used to determine: - Amount of copper in brass - Amount Fe/iron in iron pill/food - Amount H2O2 commercial peroxide solution - Amount OCI - /hypochlorite/CI2 in bleach - Amount Vitamin C - Amount Dissolve oxygen content/BOD - Amount ethanol in beer/wine - Amount oxalate acid ? MnO4 - + 5Fe2+ + 8H+→ Mn2+ + 5Fe3+ + 4H2O Cr2O7 2- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ 7H2O Iron determination using MnO4 - / Cr2O7 2- purple colourless orange green add MnO4 - till endpoint ↓ turn purple (excess MnO4 - ) add Cr2O7 2- till endpoint ↓ turn orange (excess Cr2O7 2-)
- 3. Redox Titration Calculation- % Iron in iron tablet Iron tablet contain hydrated iron (II) sulphate (FeSO4.7H2O).One tablet weighing 1.863g crushed, dissolved in water and solution made up to total vol of 250ml. 10ml of this solution added to 20ml of H2SO4 and titratedwith 0.002M KMnO4. Average 24.5ml need to reach end point. Cal % iron(II) sulphate in iron tablet. 1 1.863 g 250ml KMnO4 M = 0.002M V = 24.5 ml Fe2+ M = ? V = 30ml MnO4- + 5Fe2+ + 8H+ → Mn2+ + 5Fe2+ + 4H2O M = 0.002M M = ? V = 24.5ml Mole ratio – 1: 5 Using mole ratio Mole KMO4 - = MV = (0.002 x 0.0245) = 4.90 x 10-5 Mole ratio (1 : 5) • 1 mole KMO4 - react 5 mole Fe2+ • 4.90 x 10-5 KMO4 -react 2.45 x 10-4 Fe2+ M V = 1 M V 5 0.002 x 0.0245 = 1 Moles Fe2+ 5 Moles = 2.45 x 10-4 Fe2+ Mass of (expt yield) = 1.703g Mass of (Actual tablet) = 1.863g % Fe in iron tablet = 1.703 x 100% 1.863 = 91.4% Mole Mass Mole x RMM = Mass FeSO4 6.125 x 10-3 x 278.05 = 1.703g FeSO4 Using formula 10ml sol contain - 2.45 x 10-4 Fe2+ 250ml sol contain - 250 x 2.45 x 10-4 Fe2+ 10 = 6.125 x 10-3 mole Fe2+ FeSO4.7H2O FeSO4 + 7H2O 1 mol 1 mol + 7 mol FeSO4 Fe2+ + SO4 2- 1 mol 1mol + 1mol 6.125 x 10-3 mol 6.125 x 10-3 mole Fe2+ 1 2 3 4 Video on % Iron in iron tablet Video on Fe2+/KMnO4 titrationcalculation
- 4. Redox Titration Calculation- % Iron in iron tablet One iron tablet weighing 2.00g crushed, dissolved in water/acidto convert it to Fe2+ and solution titratedwith 0.100M KMnO4. Average 27.5ml KMnO4 needed to reachend point. Cal mass of iron and % iron in iron tablet. How equivalent point is detected ? 2 2.000 g KMnO4 M = 0.100M V = 27.5 ml Fe2+ M = ? 1MnO4 - + 5Fe2+ + 8H+ → Mn2+ + 5Fe2+ + 4H2O M = 0.100M M = ? V = 27.5ml Mole ratio – 1: 5 Using mole ratio Mole KMO4 - = MV = (0.100 x 0.0275) = 0.00275 Mole ratio (1 : 5) • 1 mole KMO4 - react 5 mole Fe2+ • 0.00275 KMO4 -react 0.01375Fe2+ M aVa = 1 Mb Vb 5 0.100 x 0.0275 = 1 Moles Fe2+ 5 Moles = 0.01375 mol Fe2+ Mass of (expt yield) = 0.7679g Mass of (Actual tablet) = 2.000g % Fe in iron tablet = 0.7679 x 100% 2.000 = 38.4 % Mole Mass Mole x RMM = Mass Fe 0.01375 x 55.85 = 0.7679g Fe Using formula 1 2 3 Video on % Iron in iron tablet Video on Fe2+/KMnO4 titrationcalculation MnO4 - – In burette is purple – Turns colourless react with Fe2+ All Fe2+ used up at equivalence point – excess KMnO4 - turn purple 4
- 5. 1 mol 1 mol 1OCI- + 2I- + 2H+ → I2 + 1CI- + H2O I2 + 2S2O3 2- → S4O6 2- + 2I- 1 mol 2 mol 10.0ml bleach (OCI -) diluted to total vol of 250ml. 20.0ml is added to 1g of KI (excess) and iodine produced is titrated with 0.0206M Na2S2O3.Using starch indicator,end point was 17.3ml. Cal molarity of OCI- in bleach. Redox Titration Calculation – OCI- in Bleach 3 Na2S2O3 M = 0.0206M V = 17.3ml I2 M = ? 2S2O3 2- + I2 → S4O6 2- + 2I- M = 0.0206 Mole = ? V = 17.3ml V = 0.02 Mole ratio (1 : 2) 1 mole OCI- : 1 mole I2 : 2 mole S2O3 2- 1 mole OCI- 2 mole S2O3 2- 10.0ml OCI- transfer V = 250ml M = 8.9 x 10-3 M 20ml transfer 1g KI excess added Mole S2O3 2- = MV = (0.0206 x 0.0173) = 3.56 x 10-4 Mole ratio (2 : 1) • 2 mole S2O3 2- react 1 mole I2 • 3.56 x 10-4 S2O3 2-- react 1.78 x 10-4 I2 Mole ratio – 2: 1 1OCI- + 2I- + 2H+ → I2 + 1CI- + H2O 1CIO- I2 Mole = ? Mole = 1.78 x 10-4 Mole ratio – 2: 1 Mole ratio (1 : 1) • 1 mole OCI- 1 mole I2 • 1.78 x 10-4 OCI- 1.78 x 10-4 I2 Moles of OCI- = M x V M x V = 1.78 x 10-4 M x 0.02 = 1.78 x 10-4 M = 1.78 x 10-4 002 M = 8.9 x 10-3 M diluted 25x Mole bef dilution= Mole aft dilution M1 V1 = M2V2 M1 = Ini molarity M2= Final molarity V1 = Initial vol V2 = Final vol M1 V1 = M2 V2 M1 x 10 = 8.9 x 10-3 x 250 M1 = 8.9 x 10-3 x 250 10 M1 = 0.222M Diuted 25x V = 10 M = ? titrated Water added till 250ml 1 Using direct formula M V (OCI+) = 1 = 1 M V (S203 2-) 2 2 Moles of OCI+ = 1 0.0206 x 0.0173 2 Moles of OCI- = 1.78 x 10-4 2 3 4 5 6 Hypochlorous acid = bleach Oxidizing agent = OCI- Iodometric titration I2/thiosulphate/starch ↓ I - oxidized by OA to I2 ↓ I2 react with starch (blue black colour) ↓ S2O3 2- added to reduce I2 ↓ I2 used up – blue black disappear 2I- + OCI- ↔ I2 + CI- 2S2O3 2- + I2 ↔S4O6 2- + 2I-
- 6. 1 mol 1 mol 1OCI- + 2I- + 2H+ → I2 + CI- + H2O I2 + 2S2O3 2- → S4O6 2- + 2I- 1 mol 2 mol 10.0ml bleach (OCI-) react with KI (excess), iodine produced is titrated with 0.020M Na2S2O3.Using starchindicator, end point was 38.65 ml. Cal molarity of OCI- in bleach. Redox TitrationCalculation – OCI- in Bleach 4 Na2S2O3 M = 0.020M V = 38.5 ml I2 M = ? 2S2O3 2- + I2 → S4O6 2- + 2I- M = 0.020 Mole = ? V = 38.55ml Mole ratio ( 1 : 2) 1 mole OCI- : 1 mole I2 : 2 mole S2O3 2- 1 mole OCI- 2 mole S2O3 2- 10ml bleach transfer 1g KI excess added Mole S2O3 2- = MV = (0.020 x 0.03865) = 7.73 x 10-4 Mole ratio (2 : 1) • 2 mole S2O3 2- react 1 mole I2 • 7.73 x 10-4 S2O3 2-- react 3.865 x 10-4 I2 Mole ratio – 2: 1 1 OCI- + 2I- + 2H+ → I2 + 2CI- + H2O 1 OCI- I2 Mole = ? Mole = 3.865 x 10-4 Mole ratio – 1: 1 Mole ratio (1 : 1) • 1 mole OCI- 1 mole I2 • 3.865 x 10-4 OCI- 3.865 x 10-4 I2 M x V = Moles OCI- M x 10 = 3.865 x 10 -4 1000 M = 0.0387M titrated 1 Using direct formula M V (OCI+) = 1 = 1 M V (S203 2-) 2 2 Moles of OCI+ = 1 0.020 x 0.03865 2 Moles of OCI- = 3.5865 x 10-4 2 3 4 5 Video on OCI- in bleach Sample OCI- calculation. Click here to view Conc OCI- Hypochlorous acid = bleach Active oxidizing agent = OCI- Iodometric titration I2/thiosulphate/starch ↓ I - oxidized by OA to I2 ↓ I2 react with starch (blue black colour) ↓ S2O3 2- added to reduce I2 ↓ I2 used up – blue black disappear 2I- + OCI- ↔ I2 + CI- 2S2O3 2- + I2 ↔S4O6 2- + 2I-
- 7. 2 mol 1 mol 2Cu2+ + 4I- → I2 + 2CuI I2 + 2S2O3 2- → S4O6 2- + 2I- 1 mol 2 mol 2.5g brass react with 10ml conc HNO3 producing Cu2+ ions. Solution made up to 250ml using water in a volumetric flask. Pipette 25ml of solution into conical flask. Na2CO3 added to neutralize excess acid. 1g KI (excess) and few drops of starch added to flask. Titrate with 0.1M S2O3 2- and end point, reachedwhen 28.2ml added. Find molarity copper ions and % copper found in brass. Redox Titration Calculation - % Cu in Brass 5 Na2S2O3 M = 0.1M V = 28.2ml I2 M = ? 2S2O3 2- + I2 → S4O6 2- + 2I- M = 0.1M Mole = ? V = 28.2ml Mole ratio ( 1 : 1) 2 mole Cu2+ : 1 mole I2 : 2 mole S2O3 2- 2 mole Cu2+ 2 mole S2O3 2- Pour into Volumetric flask V = 250ml M = ? 25ml transfer 1g KI excess + starch added Mole S2O3 2- = MV = (0.1 x 0.0282) = 2.82 x 10-3 Mole ratio (2 : 1) • 2 mole S2O3 2- react 1 mole I2 • 2.82 x 10-3 S2O3 2-- react 1.41 x 10-3 I2 Mole ratio – 2: 1 2Cu2+ + 4I- → I2 + 2CuI Mole = ? 1.41 x 10-3 I2 Mole ratio – 2: 1 Mole ratio (2 : 1) • 2 mole Cu2+ 1 mole I2 • 2.82 x 10-3 Cu2+ 1.41 x 10-3 I2 Mole of Cu2+ = M x V M x V = 2.82 x 10-3 M x 0.025 = 2.82 x 10-3 M = 2.82 x 10-3 0.025 M = 1.13 x 10-1 M Mass Cu = Molarity Cu x RAM Mass Cu = (0.113 x 63.5)g Cu in 1000ml = 7.18g Cu in 1000ml = 1.79g Cu in 250ml 10 ml HNO3 titrated Water added till 250ml 2.5g brass % Cu in brass = mass Cu x 100% mass brass = 1.79 x 100% 2.5 = 71.8% Using formulaUsing mole ratio Using formula M V (Cu2+) = 2 = 1 M V(S203 2-) 2 1 Moles of Cu2+ = 1 0.1 x 0.0282 1 Moles of Cu2+ = 2.82 x 10-3 1 2 3 4 5 6 Iodometric titration I2/thiosulphate/starch ↓ I - oxidized by OA to I2 ↓ I2 react with starch (blue black colour) ↓ S2O3 2- added to reduce I2 ↓ I2 used up – blue black disappear 4I- + 2Cu+ ↔ I2 + 2CuI 2S2O3 2- + I2 ↔S4O6 2- + 2I- Click here here for copper determination
- 8. 2 mol 1 mol 2Cu2+ + 4I- → I2 + 2CuI I2 + 2S2O3 2- → S4O6 2- + 2I- 1 mol 2 mol Brass is a copper alloy. Analysis carried out to determine copper. Iodometric titration was performed. Step 1 : Cu + 2HNO3 + 2H+ → Cu2+ + 2NO2 + 2H2O Step 2 : 4I- + 2Cu2+ → 2CuI + I2 Step 3 : I2 + 2S2O3 2- → 2I- + S4O6 2- Average vol S2O3 2- was 28.50ml. Redox Titration Calculation - % Cu in Brass 6 Na2S2O3 M = 0.1M V = 28.5ml I2 M = ? V = 100ml 2S2O3 2- + I2 → S4O6 2- + 2I- M = 0.1M Mole = ? V = 28.5ml Mole ratio ( 1 : 1) 2 mole Cu2+ : 1 mole I2 : 2 mole S2O3 2- 2 mole Cu2+ 2 mole S2O3 2- V = 100ml M = ? 1g KI excess/starch added Mole S2O3 2- = MV = (0.1 x 0.0285) = 2.85 x 10-3 Mole ratio (2 : 1) • 2 mole S2O3 2- react 1 mole I2 • 2.85 x 10-3 S2O3 2-- react 1.41 x 10-3 I2 Mole ratio – 2: 1 2Cu2+ + 4I- → I2 + 2CuI Mole = ? 1.41 x 10-3 I2 Mole ratio – 2: 1 Mole ratio (2 : 1) • 2 mole Cu2+ 1 mole I2 • 2.82 x 10-3 Cu2+ 1.41 x 10-3 I2 Mass Cu = Mole Cu x RAM Mass Cu = (2.85 x 10-3 x 63.5) g Cu = 0.181 g titrated HNO3 and water added till 100ml 0.456g brass % Cu in brass = mass Cu x 100% mass brass = 0.181 x 100% 0.468 = 39.7% Using formulaUsing mole ratio Using formula M V (Cu2+) = 2 = 1 M V(S203 2-) 2 1 Moles of Cu2+ = 1 0.1 x 0.0285 1 Mole of Cu 2+ = 2.85 x 10-3 M V (Cu2+) = 2 = 1 M V(S203 2-) 2 1 M x 0.100 = 1 0.1 x 0.0285 1 Conc Cu2+ = 2.85 x 10-2 1 2 3 4 5 6 Iodometric titration I2/thiosulphate/starch ↓ I - oxidized by OA to I2 ↓ I2 react with starch (blue black colour) ↓ S2O3 2- added to reduce I2 ↓ I2 used up – blue black disappear 4I- + 2Cu+ ↔ I2 + 2CuI 2S2O3 2- + I2 ↔S4O6 2- + 2I- Click here here for copper determination expt Cal Amt S2O3 2- Cal Conc/Mole/Mass Cu Cal % Cu by mass in brass Cal % error (Lit value = 44.2 % Cu) % error = Expt value x 100% Lit value = (44.2 – 39.7) x 100% 44.2 = 10.2%
- 9. Redox Titration Calculation- % purity of oxalate ion Purity of sodium oxalate Na2C2O4 is determine by redox titrationwith standard 0.040M KMnO4. 35.62 ml KMnO4 needed to reach end point. Cal % w/w Na2C2O4 in sample. How equivalent point is detected? 7 oxalate solution titrated 0.5116 g KMnO4 M = 0.040M V = 35.62 ml C2O4 2- M = ? 2MnO4 - + 5C2O4 2- + 16H+ → 2Mn2+ + 10CO2 + 8H2O M = 0.040M M = ? V = 35.62 ml Mole ratio – 2: 5 Using mole ratio Mole KMO4 - = MV = (0.040 x 0.03562) = 1.42 x 10-3 Mole ratio (2 : 5) • 2 mol KMO4 - react 5 mol C2O4 2- • 1.42 x 10-3 KMO4 -react 3.55 x 10-3 C2O4 2- M aVa = 2 Mb Vb 5 0.04 x 0.03562 = 2 Mole C2O4 2- 5 Mol C2O4 2- = 3.55 x 10 -3 Mass of (expt yield) = 0.476 g Mass of (Actual tablet) = 0.5116 g % w/w in Na2C2O4 = 0.476 x 100 % 0.5116 = 93 % Mole Mass Mole x RMM = Mass Na2C2O4 3.55 x 10-3 x 134 = 0.476 g Fe Using formula 1 2 3 MnO4 - – In burette is purple – Turns colourless react with C2O4 2- All C2O4 2- usedup at equivalence point – excess KMnO4 - turn purple ? Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2- KIO3 Vitamin C CIO-/Cu2+ Oxalate/ C2O4 2- MnO4 – reduced to Mn2+ C2O4 2- oxidized to CO2 (+7) ON decrease ↓ (+2) (+3) ON increase ↑ (+4) 4
- 10. M V (KIO3) = 1 M V (C6H8O6) 3 0.002 x 0.0255 = 1 Mole C6H8O6 3 Mole C6H8O6 = 1.53 x 10-4 Mole C6H8O6 = M x V M x V = 1.53 x 10-4 M x 0.025 = 1.53 x 10-4 M = 1.53 x 10-4 0025 M = 6.12 x 10-3 M 1 mol 3 mol KIO3 + 5KI + 6H+ → 3I2 + 6K+ + 3H2O 3C6H8O6 + 3I2 → 3C6H6O6 + 6I- + 6H+ 3 mol 3 mol Iodometric titrationwas performedon Vit C, (C6H8O6).25ml Vit C is titrated with 0.002M KIO3 from burette, using excess KI and starch. Average vol KIO3 is 25.5ml. Cal molarity of Vit C. Redox Titration Calculation – Vitamin C quantification 8 KIO3 M = 0.002M V = 25.5ml Vit C M = ? V = 25ml KIO3 + 5KI + 6H+ → 3I2 + 3H2O + 6K= M = 0.002M Mole = ? V = 25.5ml Mole ratio (1 :3) 1 mol KIO3 : 3 mol I2 : 3 mol C6H8O6 1 mol KIO3 3 mol C6H8O6 V = 25ml M = ? 25ml transfer 1g KI excess + starch added Mole KIO3 = MV = (0.002 x 0.0255) = 5.10 x 10-5 Mole ratio (1 : 3) • 1 mole KIO3 produce 3 mole I2 • 5.10 x 10-5 KIO3 produce 1.53 x 10-4 I2 Mole ratio – 1: 3 3C6H8O6 + 3I2 → 3C6H6O6 + 6I- + 6H+ Mole = ? 1.53 x 10-4 Mole ratio – 3: 3 Mole ratio (1 : 3) • 1 mol KIO3 react 3 mol C6H8O6 • 5.10 x 10-5 KIO3 react 1.53 x 10-4 C6H8O6 Mole C6H8O6 = M x V M x V = 1.53 x 10-4 M x 0.025 = 1.53 x 10-4 M = 1.53 x 10-4 0025 M = 6.12 x 10-3 M titrated Using mole ratio Using formula Using formula Vitamin C 1 2 3 4 Click here here to view sample Vitamin C expt ? Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2- KIO3 Vitamin C CIO-/Cu2+ Oxalate/ C2O4 2-
- 11. 25ml of undiluted H2O2 is transferto 250ml volumetric flask. (Diluted 10x ). 25ml diluted sample was titratedwith standard 0.02114M KMnO4. 28.64 ml KMnO4 needed to reach end point. Cal conc in M H2O2 sample. Assuming density is 1g/ml, calculate % H2O2 by weight. (Theoreticalvalue H2O2 = 3%) 9 25ml pipette solution KMnO4 M = 0.02114M V = 28.64 ml H2O2 M = ? 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O M = 0.02114M M = ? V = 28.64 ml Mole ratio – 2: 5 Using mole ratio Mole KMO4 - = MV = (0.02114 x 0.02864) = 6.054 x 10-4 Mole ratio (2 : 5) • 2 mol KMO4 - react 5 mol H2O2 • 6.054 x 10-4 KMO4 - react 1.513 x 10-3 H2O2 M V = 2 M V 5 0.02114 x 0.02864 = 2 Mole H2O2 5 Mol H2O2 = 1.5135 x 10 -3 Using formula 1 2 ? Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2- KIO3 Vitamin C CIO-/Cu2+ Oxalate/ C2O4 2- MnO4 – reduced to Mn2+ H2O2 oxidized to O2 (+7) ON decrease ↓ (+2) (-1) ON increase ↑ (0) Redox Titration H2O2 Calculation Pour into Volumetric flask 25 ml H2O2 Water added till 250ml Mol H2O2 = M x V M x V = 1.513 x 10-3 M x 0.025 = 1.513 x 10-3 M = 1.513 x 10-3 0.025 M = 0.06052 M (Diluted sample) Original sample = 0.06052 x 10 = 0.6052 M Conc H2O2 = 0.6052M RMM H2O2 = 34 Mass H2O2 = 0.6052 x 34 = 20.60g in 1000 ml = 2.06g in 100ml = 2.06% 3 Stronger oxidizing agent reduce weaker oxidizing agent
- 12. Cr2O7 2- reduced to Cr3+ C2H5OH oxidized CH3COOH3 % C2H5OH by mass = mass C2H5OH x 100% mass blood = 0.351 x 100% 10.0 = 3.51 % Alcohol in blood can be determined by redox titration with K2Cr2O7 3C2H5OH + 2Cr2O7 2- + 16H+ → 3CH3COOH3 + 4Cr 3+ + 11H2O Calculate % by mass of ethanol. Explain how end point is determined? 10 Cr2O7 2- M = 0.055M V = 9.25 ml C2H5 OH M = ? 2Cr2O7 2- + 3C2H5OH + 16H+ → 3CH3COOH3 + 4Cr 3+ + 11H2O M = 0.0550 M = ? V = 9.25ml Mole ratio – 3: 2 Using mole ratio Mole Cr2O7 -2- = MV = (0.055 x 0.00925) = 5.08 x 10-4 Mole ratio (2 : 3) • 2 mol Cr2O7 2- react 3 mol C2H5OH • 5.08 x 10-4 Cr2O7 2- react 7.63 x 10-3 C2H5OH M V = 2 M V 3 0.055 x 0.0925 = 2 MV 3 Mol C2H5OH = 7.63 x 10 -3 Using formula 1 2 (+7) ON decrease ↓ (+3) (-2) ON increase ↑ (0) Redox Titration Alcohol Calculation C2H5OH 10g of blood sample Mass C2H5OH = Mol x RAM Mass = 7.63 x 10-3 x 46 Mass = 0.351 g 3 Click here practical breath analyzer using dichromate Alcohol C2H5OH Ethanoic acid CH3COOH Cr2O7 2- – In burette is orange– Turns green react with C2H5OH All C2H5OH used up at equivalencepoint – excess Cr2O7 2- turn orange oxidized Dichromate Cr2O7 2- Chromate Cr3+ reduced Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2- KIO3 Vitamin C CIO-/Cu2+ Ethanol/ C2H4OH ? 4
- 13. BiologicalOxygenDemand Measure amt dissolve oxygen needed by aerobic organismto break down • Organicmatter in water sample over 5 day period • BOD polluted water – Amt dissolve oxygen need for biological decomposition • Measure amt O2 used for biochemical decomposition of organic matter • Measure amt O2 used to oxidize organic to produce energy for microbesLots of organic decomposition(uses O2) ↓ Dissolve oxygen Low ↓ used up ↓ BiologicalOxygen Demand High ↑ ↓ Level Pollution is HIGH ↑ ↓ Aquaticlife die /Toxic Low Dissolve Oxygen, signify high O2 demand from microbes (organic waste contamination) Breakdown organic matter in water consumes oxygen by aerobic micro-organisms. BOD High ↑ Dissolve O2 Low ↓ (O2 used up) Level Pollution HIGH ↑ Organic waste decomposition ↑ Aquaticlife die/Toxic BOD Low ↓ Dissolve O2 High ↑ (O2 high) Level Pollution LOW ↓ Organic waste decomposition ↓ Aquaticlife thrive BOD ↑ – No good BOD ↓ - Good Dissolve oxygen Level - • Indicator of clean water • Level of pollution BOD ↓ Dissolve Oxygen ↑ Click here carolina Winkler method BOD Click here dissolve oxygen video
- 14. Water Quality Clean Lightly polluted Moderate polluted Severely polluted Dissolve O2, mg/ml DO > 6.5 4.5 – 6.5 2.0 – 4.5 < 2.0 BOD, mg/ml < 3 3 – 4.9 5 – 15 > 15 Explosive growth algae/bloom Block sunlight for photosynthesis Eutrophicationon BOD Excessive use fertiliserslike phosphates/nitrates Wash into river/water Eutrophication Explosive growth algae/bloom ↓ When die - organic decomposition by bacteria ↓ Uses up dissolve oxygen ↓ BOD demand HIGH ↑ ↓ Water polluted Algae bloom Dissolve oxygen Low ↓ BOD High ↑ Nutrient leach BiologicalOxygenDemand Redox titration(WinklerMethod) measure dissolveO2 BOD index Click here on Winkler titration method Iodometric titration I2/thiosulphate/starch ↓ Mn2+ oxidized by O2 to Mn4+ ↓ Mn4+ oxidized I- to I2 I2 react with starch (blue black colour) ↓ S2O3 2- added to reduce I2 ↓ I2 used up – blue black disappear Measure BOD Iodometric titration
- 15. BiologicalOxygenDemand Redox titration(WinklerMethod) measure dissolveO2 BOD index 1 mol 2 mol 2Mn2+ + O2 + 4OH- → 2MnO2 + 2H2O 2MnO2 + 4I- + 4H+ → 4I2 + 2Mn2- + 4H2O 4I2 + 4S2O3 2- → 4I- + 2S4O6 2- Click here on Winkler titration method Water Quality Clean Lightly polluted Moderately polluted Severely polluted Dissolve O2, mg/ml DO > 6.5 4.5 – 6.5 2.0 – 4.5 < 2.0 BOD, mg/ml < 3 3 – 4.9 5 – 15 > 15 Dissolve O2 reacts with alkaline manganese (Mn2+) to form (Mn4+) 4Mn2+ + 4OH- → 2Mn(OH)2 1 mol 2 mol 2Mn(OH)2 + O2 → 2MnO(OH)2 2MnO(OH)2 + 8H+ + 6I- → 2I3 - + 6H2O 2 mol 2 mol 2I3 - + 4S2O3 2- → 6I- + 2S4O6 2- 2 mol 4 mol Redox titration Winkler Method DO bottle Mn2+ salt 1g KI excess alkaline/OH- shake White ppt Mn(OH)2 Conc H2SO4 White ppt dissolve in acid Na2S2O3 M = 0.05M V = 12.5ml titrated S2O3 2- 1 O2 + 4S2O3 2- → products M = ? M = 0.05M V = 12.5ml I- oxidized to I2 by Mn2+ O2 M = ? V = 500ml 2 mol 4 mol 4 mol 4 mol Mole ratio O2 : S2O3 2- (1 : 4) 1 mol O2 : 4 mol I2 : 4 mol S2O3 2- 1 mol O2 4 mol S2O3 2- Brown I2 sol form Starch added Iodometric titration I2/thiosulphate/starch ↓ Mn2+ oxidized by O2 to Mn4+ ↓ Mn4+ oxidized I- to I2 I2 react with starch (blue black colour) ↓ S2O3 2- added to reduce I2 ↓ I2 used up – blue black disappear Water sample added 1 mol O2 : 4 mol S2O3 2-
- 16. 1 mol 2 mol 2Mn2+ + O2 + 4OH- → 2MnO2 + 2H2O 2MnO2 + 4I- + 4H+ → 4I2 + 2Mn2- + 4H2O 4I2 + 4S2O3 2- → 4I- + 2S4O6 2- DissolveO2 reacts with alkalinemanganese(Mn2+) to form (Mn4+) Redox titration Winkler Method DO bottle Mn2+ salt 1g KI excess alkaline/OH- shake White ppt Mn(OH)2 Conc H2SO4 White ppt dissolve in acid Na2S2O3 M = 0.05M V = 12.5ml titrated S2O3 2- 1 O2 + 4S2O3 2- → product M = ? M = 0.05M V = 12.5ml I- oxidized to I2 by Mn2+ O2 M = ? V = 500ml 2 mol 4 mol 4 mol 4 mol Mole ratio O2 : S2O3 2- (1 : 4) 1 mol O2 : 4 mol I2 : 4 mol S2O3 2- 1 mol O2 4 mol S2O3 2- Brown I2 sol form Starch added Iodometric titration I2/thiosulphate/starch ↓ Mn2+ oxidized by O2 to Mn4+ ↓ Mn4+ oxidized I- to I2 I2 react with starch (blue black colour) ↓ S2O3 2- added to reduce I2 ↓ I2 used up – blue black disappear Water sample added 500ml water tested for dissolve oxygen by adding Mn2+ in alkaline solution, followed by addition of KI and acid. I2 produced is reduced by titrating with 0.05M S2O3 2-.Average vol S2O3 2- used is 12.50ml. Calculate dissolved oxygen in g/dm3. 1 Mole S2O3 2- = MV = (0.05 x 0.0125) = 6.25 x 10-4 Mole ratio (1 : 4) • 1 mole O2 react 4 mole S2O3 2- ? 6.25 x 10-4 S2O2 2- 6.25 x 10-4 = 1.56 x 10-4 4 1 mol O2 : 4 mol S2O3 2- 2 3 Mole O2 = 1.56 x 10-4 mol Mass O2 = Mole O2 x RAM Mass O2 = (1.56 x 10-4 x 32.0)g = (5.00 x 10-3)g in 500ml = 0.01 g in 1000ml = 0.01g/dm3 4 Click here on Winkler titration methodClick here on Winkler titration method
- 17. Titrationfor IA (DCP) assessment Acid Base Titration StandardizationHCI with primary std Na2CO3 Click here for expt 4.2 StandardizationNaOH with primary std KHP Click here or here for expt` Titrationbet NaOH with std HCI Click here for expt 4.2a Titrationbet HCI with std NaOH Click here for expt 4.2a Determiningwater crystallization in hydratedNa2CO3 with std HCI Click here for expt 4.4 StandardizationKMnO4 with std ammonium iron(II)sulphate Click here for expt 4.5 Iron (II) determination with std KMnO4 Click here for expt 4.6 Hypochlorite(OCI-) in bleach with iodine/thiosulphate Click here for expt 4.8 Determiningethanoicacid in vinegarwith std NaOH Click here for expt 4.3 Copper(II)determination in brass with iodine/thiosulphate Click here or here for expt` Click here for more expt StandardizationKI/I2 with std KIO3 Click here for expt 4.7 Click here for more expt Determiningacetylsalicylicacid in aspirinwith std NaOH Click here or here for expt` Click here for more expt Vit C determinationwith iodine/thiosulphate Click here or here for expt Click here more detail expt StandardizationExpt Acid/Base Titration Expt StandardizationExpt Redox Titration Expt Redox Titration Standardization KI/I2 with std sodium thiosulphate Click here for expt 4.7 Iodine/thiosulphate(iodometrictitration)
- 18. CI2 + 2KBr-→ 2KCI + Br2 3CuO + 2NH3→ 3H2O+ 3Cu + N2 Redox (Oxidationand Reduction) (+7) (+2)Mn red - ON ↓ (+2) Fe oxi – ON ↑ (+3) MnO4 - + Fe2+ + 8H+ → Mn2+ + Fe3+ 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reducing Agent MnO4 - Fe2+ Reduction Oxidation Oxidizing Agent Reducing Agent CI2 Br- Reduction Oxidation Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation (0) CI red – ON ↓ (-1) (-1) Br - oxi – ON ↑ (0) Oxidizing Agent Reducing Agent CuO NH3 Reduction Oxidation Reducing agent ↓ Oxidation (-3) NH3 oxi – ON ↑ (0) Oxidizing agent ↓ Reduction (+2) Cu red – ON ↓ (0) 2HCI + Zn → H2 + ZnCI2 (0) Zn oxi – ON ↑ (+2)Reducing agent ↓ Oxidation Oxidizing agent ↓ Reduction (+1) H red – ON ↓ (0) Oxidizing Agent Reducing Agent HCI Zn Reduction Oxidation
- 19. CI2 + 2KBr-→ 2KCI + Br2 3CuO + 2NH3→ 3H2O+ 3Cu +N2 Redox (Oxidationand Reduction) (+7) (+2)Mn red - ON ↓ (+2) Fe oxi – ON ↑ (+3) MnO4 - + 8H+ + Fe2+ → Mn2+ + Fe3+ 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation (0) CI red – ON ↓ (-1) (-1) Br - oxi – ON ↑ (0) Reducing agent ↓ Oxidation (-3) NH3 oxi – ON ↑ (0) Oxidizing agent ↓ Reduction (+2) Cu red – ON ↓ (0) 2HCI + Zn → H2 + ZnCI2 (0) Zn oxi – ON ↑ (+2)Reducing agent ↓ Oxidation Oxidizing agent ↓ Reduction (+1) H red – ON ↓ (0) Reducing Agent Oxidation Fe 2+ → Fe2+ + e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Reducing Agent Oxidation 2Br - → Br2 + 2e- Loss electron Increase ON ↑ Oxidizing Agent Reduction CI2 + 2e → 2CI- Gain electron Decrease ON ↓ Reducing Agent Oxidation (NH3) -N3- → N + 3e- Loss electron Increase ON ↑ Oxidizing Agent Reduction (CuO) Cu2+ + 2e → Cu Gain electron Decrease ON ↓ Reducing Agent Oxidation Zn → Zn2+ + 2e- Loss electron Increase ON ↑ Oxidizing Agent Reduction 2H+ + 2e → H2 Gain electron Decrease ON ↓
- 20. Redox (Oxidationand Reduction) Half equations Oxidation rxn Oxidation half eqn Reduction half eqn Loss electron ↓ Reduction rxn Loss hydrogen ↓ Gain oxygen ↑ Gain ON ↑ Gain electron ↑ Gain hydrogen ↑ Loss oxygen ↓ Loss ON ↓ OxidizingAgentReducing Agent Oxidation rxn Reduction rxn lose electron Zn + 2H+ → H2 + Zn2+ Zn → Zn2+ + 2e 2H+ + 2e → H2 (0) ON increase ↑ (+2) Zn → Zn2+ + 2e 2H+ + 2e → H2 2H+ + Zn → Zn2+ + H2 lose electron gain electron (+1) ON decrease ↓ (0) Completefull eqn Zn + Cu2+ → Zn2+ + CuOxidation half eqn Zn → Zn2+ + 2e lose electron (0) ON increase ↑ (+2) Reduction half eqn Cu2+ + 2e → Cu (+2) ON decrease ↓ (0) gain electron Zn → Zn2+ + 2e Cu2+ + 2e → Cu Cu2+ + Zn → Zn2+ + Cu Half equations
- 21. Redox (Oxidationand Reduction) Half equations Oxidation half eqn Reduction half eqn Zn → Zn2+ + 2e 2H+ + 2e → H2 (0) ON increase ↑ (+2) Zn → Zn2+ + 2e 2H+ + 2e → H2 2H+ + Zn → Zn2+ + H2 lose electron gain electron (+1) ON decrease ↓ (0) Completefull eqn Oxidation half eqn Zn → Zn2+ + 2e lose electron (0) ON increase ↑ (+2) Reduction half eqn Cu2+ + 2e → Cu (+2) ON decrease ↓ (0) gain electron Zn → Zn2+ + 2e Cu2+ + 2e → Cu Cu2+ + Zn → Zn2+ + Cu Half equations Zn + 2HCI → H2 + ZnCI2 Zn + 2H+ + 2CI- → H2 + Zn2+ + 2CI - Completeionic/redox eqn Zn + 2H+ → H2 + Zn2+ spectator ionsspectator ions Zn + 2H+ → H2 + Zn2+ Zn + CuSO4 → ZnSO4 + Cu Zn + Cu2++ SO4 2- → Zn2+ + SO4 2- + Cu Completefull eqn Completeionic/redox eqn spectator ions Zn + Cu2+ → Zn2+ + Cu Half equations Half equations Zn + Cu2+ → Zn2+ + Cu
- 22. Redox (Oxidationand Reduction) Half equations Oxidation half eqn Reduction half eqn Mg → Mg2+ + 2e Pb2+ + 2e → Pb (0) ON increase ↑ (+2) Mg → Mg2+ + 2e Pb2+ + 2e → Pb Pb2+ + Mg → Mg2+ + Pb lose electron gain electron (+2) ON decrease ↓ (0) Completefull eqn Oxidation half eqn 2Br- → Br2 + 2e lose electron (-1) ON increase ↑ (0) Reduction half eqn CI2 + 2e → 2CI- (0) ON decrease ↓ (-1) gain electron 2Br- → Br2 + 2e CI2 + 2e → 2CI- CI2 + 2Br- → 2CI- + Br2 Half equations Mg + PbO → Pb + MgO Mg + Pb2+ + O2- → Pb + Mg2+ + O 2- Completeionic/redox eqn spectator ionsspectator ions Mg + Pb2+ → Pb + Mg2+ 2KBr + CI2 → Br2 + 2KCI 2K+ + 2Br- + CI2 → Br2 + 2K+ + 2CI - Completefull eqn Completeionic/redox eqn spectator ions 2Br- + CI2 → Br2 + 2CI- Half equations Half equations Mg + Pb2+ → Pb + Mg2+ 2Br- + CI2 → Br2 + 2CI- lose electron
- 23. MnO4 - + 8H+ + 5Fe2+ → Mn2+ + 5Fe3+ + 4H2O ConstructingHalf and complete redox equation (+7) (+2)Mn red - ON ↓ (+2) Fe oxi – ON ↑ (+3) MnO4 - + 5Fe2+ + 8H+ → Mn2+ + 5Fe3+ + 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Reducing Agent Oxidation Fe 2+ → Fe2+ + e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O -add H2O 2. Balance# H add H+ 3. Balance# charges -add electrons 4. Balance# electron transfer MnO4 - → Mn2+ MnO4 - → Mn2+ + 4H2O MnO4 - + 8H+ → Mn2++ 4H2O MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O Fe2+ → Fe3+ Fe2+ → Fe3+ + e- 5Fe2+ → 5Fe3+ + 5e-MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O x 5x 1 MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O 5Fe2+ → 5Fe3+ + 5e- + MnO4 - - In acidic medium - Strong oxidizing agent MnO4 - + 8H+ + 5Fe2+ → Mn2+ + 5Fe3+ 4H2O
- 24. 2MnO4 - + 5SO2+ 2H2O → 2Mn2+ + 5SO4 2- + 4H+ ConstructingHalf and complete redox equation (+7) (+2)Mn red - ON ↓ (+4) SO2 oxi – ON ↑ (+6) 2MnO4 - + 5SO2 + 2H2O→ 2Mn2+ + 5SO4 2- + 4H+ Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Reducing Agent Oxidation SO2 → SO4 2- + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer MnO4 - → Mn2+ MnO4 - → Mn2+ + 4H2O MnO4 - + 8H+ → Mn2++ 4H2O MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O SO2 → SO4 2- 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O x 5x 2 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O 5SO2 + 10H2O → 5SO4 2- + 20H+ + 10e- + 2MnO4 - + 5SO2 + 2H2O→ 2Mn2+ + 5SO4 2- 4H+ SO2 + 2H2O → SO4 2- SO2 + 2H2O → SO4 2- + 4H+ SO2 + 2H2O → SO4 2- + 4H+ + 2e- 5SO2 + 10H2O → 5SO4 2- + 20H+ + 10e-
- 25. 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O ConstructingHalf and complete redox equations (+7) (+2)Mn red - ON ↓ (-1) H2O2 oxi – ON ↑ (0) 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction MnO4 - + 5e → Mn2+ Reducing Agent Oxidation H2O2 → O2 + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer MnO4 - → Mn2+ MnO4 - → Mn2+ + 4H2O MnO4 - + 8H+ → Mn2++ 4H2O MnO4 - + 8H+ + 5e- → Mn2+ + 4H2O 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O x 5x 2 2MnO4 - + 16H+ + 10e- → 2Mn2+ + 8H2O 5H2O2 → 5O2 + 10H+ + 10e- + 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O H2O2 → O2 H2O2 → O2 + 2H+ H2O2 → O2 + 2H+ + 2e- 5H2O2 → 5O2 + 10H+ + 10e-
- 26. Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O Cr2O7 2-→ 2Cr3+ ConstructingHalf and complete redox equations (+6) (+3)Cr red - ON ↓ (+3) NO2 - oxi – ON ↑ (+5) Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction Cr2O7 2- + 6e- → 2Cr3+ Reducing Agent Oxidation NO2 - → NO3 - + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 1 Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O 3NO2 -+ 3H2O → 3NO3 - + 6H+ + 6e- + Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O Cr2O7 2- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O NO2 - → NO3 - NO2 - + H2O → NO3 - NO2 - + H2O → NO3 - + 2H+ NO2 - + H2O → NO3 - + 2H+ + 2e- 3NO2 - + 3H2O → 3NO3 - + 6H+ + 6e-
- 27. Cr2O7 2- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ + 7H2O Cr2O7 2-→ 2Cr3+ ConstructingHalf and complete redox equations (+6) (+3)Cr red - ON ↓ (+2) Fe2+ oxi – ON ↑ (+3) Cr2O7 2- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ + 7H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction Cr2O7 2- + 6e- → 2Cr3+ Reducing Agent Oxidation Fe2+ → Fe3+ + e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 6x 1 Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O 6Fe2+ → 6Fe3+ + 6e- + Cr2O7 2- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O Cr2O7 2- + 14H+ + 6e- → 2Cr3+ + 7H2O Cr2O7 2- + 6Fe2+ + 14H+ → 2Cr3+ + 6Fe3+ 7H2O Fe2+ → Fe3+ Fe2+ → Fe3+ + e 6Fe2+ → 6Fe3+ + 6e
- 28. ConstructingHalf and complete redox equations (+5) (-1)CIO3 - red - ON ↓ (-1) I- oxi – ON ↑ (0) CIO3 - + 6I- + 6H+ → 3I2 + CI- + 3H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction CIO3 - + 6e- → CI- Reducing Agent Oxidation 2I- → I2 + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 1 CIO3 - + 6H+ + 6e- → CI- + 3H2O 6I- → 3I2 + 6e- + CIO3 - + 6I- + 6H+ → 3I2 + CI- + 3H2O CIO3 - → CI- CIO3 - → CI- + 3H2O CIO3 - + 6H+ → CI- + 3H2O CIO3 - + 6H+ + 6e- → CI- + 3H2O CIO3 - + 6H+ + 6e- → CI- + 3H2O 2I- → I2 2I- → I2 + 2e- 6I- → 3I2 + 6e- CIO3 - + 6H++ 6I- → 3I2 + 3H2O
- 29. ConstructingHalf and complete redox equations (+5) (+2)NO3 - red - ON ↓ (0) Cu oxi – ON ↑ (+2) 2NO3 - + 3Cu + 8H+ → 3Cu2+ + 2NO + 4H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction NO3 - + 3e- → NO Reducing Agent Oxidation Cu → Cu2+ + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 2 2NO3 - + 8H+ + 6e- → 2NO + 4H2O 3Cu → 3Cu2+ + 6e- + 2NO3 - + 3Cu + 8H+ → 3Cu2+ + 2NO + 4H2O NO3 - → NO NO3 - → NO + 2H2O NO3 - + 4H+ → NO + 2H2O NO3 - + 4H+ + 3e- → NO + 2H2O 2NO3 - + 8H+ + 6e- → 2NO + 4H2O Cu → Cu2+ Cu → Cu2+ + 2e- 3Cu → 3Cu2+ + 6e- 2NO3 - + 8H+ + 3Cu → 3Cu2+ +2NO + 4H2O
- 30. HNO3 +3Fe2+ + 3H+ → 3Fe3+ + NO + 2H2O ConstructingHalf and complete redox equations (+5) (+2)HNO3 red - ON ↓ (+2) Fe oxi – ON ↑ (+3) HNO3 + 3Fe2+ + 3H+ → 3Fe3+ + NO + 2H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction HNO3 + 3e- → NO Reducing Agent Oxidation Fe 2+ → Fe3++ e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 3x 1 HNO3 + 3H+ + 3e- → NO + 2H2O 3Fe2+ → 3Fe3+ + 3e- + HNO3 → NO + 2H2O HNO3+ 3H+ → NO + 2H2O HNO3 + 3H+ + 3e- → NO + 2H2O HNO3 + 3H+ + 3e- → NO + 2H2O Fe2+ → Fe3+ HNO3 + 3Fe2+ + 3H+ → 3Fe3+ + NO + 2H2O HNO3 → NO Fe2+ → Fe3+ + e- 3Fe2+ → 3Fe3+ + 3e-
- 31. H2O2 + 2Fe2+ +2H+ → 2Fe3+ + 2H2O ConstructingHalf and complete redox equations (-1) (-2)H2O3 red - ON ↓ (+2) Fe oxi – ON ↑ (+3) H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 2H2O Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction H2O3 + e- → H2O Reducing Agent Oxidation Fe 2+ → Fe3++ e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer x 2x 1 H2O2 + 2H+ + 2e- → 2H2O 2Fe2+ → 2Fe3+ + 2e- + Fe2+ → Fe3+ Fe2+ → Fe3+ + e- 2Fe2+ → 2Fe3+ + 2e- H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 2H2O H2O2 → H2O H2O2 → 2H2O H2O2 + 2H+ → 2H2O H2O2 + 2H+ + 2e- → 2H2O H2O2 + 2H+ + 2e- → 2H2O
- 32. CI2 + SO2 + 2H2O → 2CI- + SO4 2- + 4H+ ConstructingHalf and complete redox equations (0) (-1)CI2 red - ON ↓ (+4) SO2 oxi – ON ↑ (+6) CI2 + SO2 + 2H2O→ 2CI- + SO4 2- + 4H+ Oxidizing agent ↓ Reduction Reducing agent ↓ Oxidation Oxidizing Agent Reduction CI2 + 2e → 2CI- Reducing Agent Oxidation SO2 → SO4 2- + 2e- Loss electron Increase ON ↑ Gain electron Decrease ON ↓ Completefull eqn Oxidation half eqnReduction half eqn 1. Balance # O - add H2O 2. Balance# H add H+ 3. Balance# charges - add electrons 4. Balance# electron transfer SO2 → SO4 2- x 1x 1 CI2 + 2e- → 2CI- SO2 + 2H2O → SO4 2- + 4H+ + 2e- + SO2 + 2H2O → SO4 2- SO2 + 2H2O → SO4 2- + 4H+ SO2 + 2H2O → SO4 2- + 4H+ + 2e- CI2 + SO2 + 2H2O→ 2CI- + SO4 2- + 4H+ CI2 → 2CI- CI2 + 2e- → 2CI- CI2 + 2e- → 2CI- SO2 + 2H2O → SO4 2- + 4H+ + 2e-
- 33. Sn2+ + 2Fe3+ → Sn4+ + 2Fe2+2Fe2+ + CI2 → 2Fe3+ + 2CI-Ca + 2H+ → Ca2+ + H2 IB Redox Questions Deduce half eqn of oxidation and reduction for the following Ca + 2H+ → Ca2+ + H2 2Fe2+ + CI2 → 2Fe3+ + 2CI- Sn2+ + 2Fe3+ → Sn4+ + 2Fe2+ 0 +1 +2 0 Ca → Ca2+ + 2e 2H+ + 2e → H2 oxidation reduction +2 0 +3 -1 2Fe2+ → Fe3+ + 2e CI2 + 2e → 2CI- oxidation reduction +2 +3 +4 +2 Sn2+ → Sn4+ + 2e 2Fe3+ + 2e → 2Fe2+ Substancesacting as oxidizingand reducing agent 2MnO4 - + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O H2O2 + 2Fe2+ + 2H+ → 2Fe3+ + 2H2O H2O2 + 2I- + 2H+ → I2 + 2H2O Oxidizing Agent Reducing Agent MnO4 - Fe2+ Cr2O7 2- SO2 HNO3 I- H2O2 H2S CI2 SO3 2- Acidified H2O2 act as oxidizing agent - Oxidizes Fe2+ to Fe3+ - Oxidizes I- to I2 Acidified MnO4 - act as more powerful oxidizing agent - Oxidizes weaker oxidizing agent H2O2 to H2O and O2 - H2O2 act as reducing agent Identify oxidizingand reducing agentfor followingrxn. 5As2O3 + 2MnO4 - + 16H+ → 2Mn2+ + 5As2O5 + 8H2O 2NO3 - + 3Cu + 8H+ → 3Cu2+ + 2NO+ 4H2O Cr2O7 2- + 3NO2 - + 8H+ → 2Cr3+ + 3NO3 - + 4H2O 1 2 3 oxidizing agent oxidizing agent oxidizing agent reducing agent reducing agent reducing agent
