Lecture13.pdfddffgggghhhbnhhhhgfdfrfghgffgf
About The Cover The front cover is an image by Eric J. Heller depicting electron flow over a microscopically bumpy surface. The paths of the 100,000 electrons b"gin at the upper right but branch and fold back on one another in a surprising pattern as they spread toward the lower left. Electron flow is the subject of several chapters in this book and is especially important in the discussion of electric sparks. Some sparks are amusing, such as those generated when someone chomps down on a wintergreen LifeSaver-the brief blue glow that illuminates the mouth can be seen in a dark room (Chapter 21). Other sparks are quite dangerous, such as those in electrostatic discharges that can accidentally cause airborne dust to explode (Chapter 25). Take Your Seat-The Show's About To Begin! This 8th edition of Fundamentals of Physics includes hundreds of items about curious effects in the everyday world, written in the spirit of Jearl Walker's The Flying Circus of Physics. The original edition of The Flying Circus of Physics-in print for over 30 years in l0languages-is a cult classic among physics students, physics instructors, and the general prblic. Electronic links to the new 2nd Edition of The Flying Circus of Physics are available in the electronic version of 8th edition of Fundamentals of Physics that is part of WileyPLUS, one of the online homework systems available with this book. WileyPLUS also includes electronic versions of all the end-of-chapter problems in Fundamentals of Physics and the interactive tutorials (several hundred) and hints (several thousand) written by author Jearl Walker. You can find out more about The Flying Circus of Physics at www.fl yi n g ci rcu sofp hysics.co m. Get A Better 6rade ln Physics! lntroductory Physics With Calculus As A Second Language by Thomas E. Barrett (0-47 1- 7391 0-3) helps you understand the basic concepts, break down problems into simple steps, and improve your problem-solving skills! Find out more at www.wiley.com/collegeibarret. @WILEY I 8O7.2OO7 KNOWLEDGE FOR GENERATIONS www.wil"y.com /col leg e/hallidryAbout The Cover The front cover is an image by Eric J. Heller depicting electron flow over a microscopically bumpy surface. The paths of the 100,000 electrons b"gin at the upper right but branch and fold back on one another in a surprising pattern as they spread toward the lower left. Electron flow is the subject of several chapters in this book and is especially important in the discussion of electric sparks. Some sparks are amusing, such as those generated when someone chomps down on a wintergreen LifeSaver-the brief blue glow that illuminates the mouth can be seen in a dark room (Chapter 21). Other sparks are quite dangerous, such as those in electrostatic discharges that can accidentally cause airborne dust to explode (Chapter 25). Take Your Seat-The Show's About To Begin! This 8th edition of Fundamentals of Physics includes hundreds of items about curious es
Recommended
More Related Content
Similar to Lecture13.pdfddffgggghhhbnhhhhgfdfrfghgffgf
Similar to Lecture13.pdfddffgggghhhbnhhhhgfdfrfghgffgf (20)
Recently uploaded
Recently uploaded (20)
Lecture13.pdfddffgggghhhbnhhhhgfdfrfghgffgf
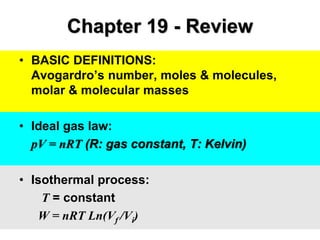
- 1. Chapter 19 - Review • BASIC DEFINITIONS: Avogardro’s number, moles & molecules, molar & molecular masses • Ideal gas law: pV = nRT (R: gas constant, T: Kelvin) • Isothermal process: T = constant W = nRT Ln(Vf /Vi)
- 2. • Chapter 19: How are ( p, V, T ) related to the motions of the atoms or molecules of the gas? • This molecular approach is called the KINETIC THEORY of GASES. • What is the relation between pressure and the speed of the molecules of the gas?
- 3. (19-3) The RMS speed • Consider a gas of n moles (N molecules) confined to a cubical box of edge length L (volume V = L3) and held at temperature T. • What is the relation between the pressure and the speed of the molecules? • The molecules move randomly in all directions. • Assumptions: 1. Ignore the collisions between the molecules. 2. Consider the collisions with the walls to be elastic.
- 6. Gas Molar Mass (g/Mol) RMS Speed (m/s) Hydrogen 2.02 1920 Helium 4.0 1370 Water Vapor 18.0 645 Nitrogen 28.0 517 Oxygen 32.0 483 Carbon Dioxide 44.0 412 Sulfur Dioxide 64.1 342 RMS Speeds of Some Gases at Room Temperature (25 oC)
- 9. Problem 2 – Book Twenty two particles have speeds as follows: Ni 2 4 6 8 2 vi(cm/s) 1 2 3 5 6 (a)What is their average speed? (b)What is their vrms? vi 2 1 4 9 25 36 ( )2
- 10. Problem 44 – Book At 546 K and 2.00 x 10-2 atm, the density of a gas is 1.24 x 10-5 g/cm3. a) Find vrms for the gas molecules. b) Find the molar mass of the gas. T p r