Many electrons atoms_2012.12.04 (PDF with links
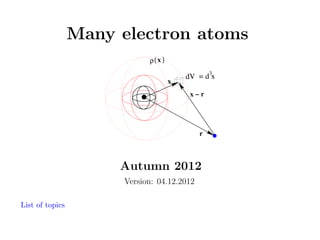
- 1. Many electron atoms ρ( x ) x 3 dV = d x x−r r Autumn 2012 Version: 04.12.2012 List of topics
- 2. (1) Pauli-principle Hund’s rule Antisymmetric functions for n-particles - Slater Determinants Filling Shells Figure Ionization energies - Figure Hartree - Selfconsistent field - Iterations only Iterations part Selfconsistent field Result - Figures Configurations - Ionization potentials of atoms - Tables Screened potential and Centrifugal barrier - Explains Tables Energy for N-particles - Using Slater Determinants Helium example Lithium example Counting nonzero terms N particles - Energy Summary Schr¨dinger equation from variational method o Variational method - deriving Hartree-Fock Equations Hartree-Fock Equations Total energy and the selfconsistent orbital energies Evaluation of electron repulsion Configuration mixing 2 1 3 2 2 8 4 3 3 2 5 1 2 1 1 2 1 3 2 slide + 3 slides slides slides slides slides slides slides tables slides slides slide slides slide slide slides slide slides slides + 2slides
- 3. 1 Filling up the shells with electrons In order to know and to, more or less, understand in which state an electron is bound we can use some basic rules. The generel idea is that the lowest energy-state is the most stable one. The exited states can in most cases fall in the lower state or ground state by emission of light. The principles one need to know how to fill up the shells are the Pauli-principle and the Hund’s rule. List of topics 3
- 4. 2 Pauli-principle The filling of the shells observed was explained by Pauli by formulating the Pauli exclusion principle: Two electrons can not be in the same state - defined here by possible quantum numbers n, l, m and ms n, the principal quantum number of the shell, l, the angular momentum quantum number of the subshell, m, the magnetic quantum number ms denotes spin ”up” and spin ”down”, ms = 1/2 or ms = −1/2 The Pauli principle mathematical: require the multiparticle wavefunction antisymmetric with respect to exchange of two particles. If two particles coordinates are exchanged, the function must change sign. If two particles are in the same state (function), this requirement leads to zero wavefunction, thus impossible. List of topics 4
- 5. Figure 1: Atomic levels (shells)List of topics 5
- 6. Figure 2: Atomic levels (shells) filled up to the element ....List of topics 6
- 7. 3 Antisymmetric product function for n-particles (−1)P (perm(α,β,...ν)) perm (φα φβ ....φν ) (x1 )(x2 )....(xn ) Ψ(x1 , x2 , ...xn ) = perm(α,β,...ν) where each term in the sum looks as φβ (x1 )...φν (x2 )...φα ..., summing over all permutations, and P (perm(α, β, ...ν)) is the number of swaps of the given permutation perm(α, β, ...ν) This is very close to the definition of the determinant n det(A) = sgn(σ) Ai,σ(i) i=1 σ∈Sn The above in this notation n Ψ(x1 , x2 , ...xn ) = sgn(σ) σ∈Sn List of topics 7 φασ(i) (xi ) i=1
- 8. Slater determinant The antisymmetric combination for n-particles can be written as a determinant in this way: φα (x1 ) φα (x2 ) ... φα (xn ) φβ (x1 ) φβ (x2 ) ... φβ (xn ) ... ... ... ... φν (x1 ) φν (x2 ) ... φν (xn ) For 3 particles: 3 particle Slater determinant φα (x1 ) φα (x2 ) φα (x3 ) φβ (x1 ) φβ (x2 ) φβ (x3 ) φγ (x1 ) φγ (x2 ) φγ (x3 ) List of topics 8
- 9. 3 × 3 determinant φα (1) φα (2) φα (3) φβ (1) φβ (2) φβ (3) φα (1) φα (2) φα (3) φβ (1) φβ (2) φβ (3) φγ (1) φγ (2) φγ (3) φγ (1) φγ (2) φγ (3) φα (1) φα (2) φα (3) φβ (1) φβ (2) φβ (3) φα (1) φβ (2) φγ (3) + − φγ (1) φβ (2) φα (3) φβ (1) φγ (2) φα (3) + − φα (1) φγ (2) φβ (3) List of topics 9 φγ (1) φα (2) φβ (3) − φβ (1) φα (2) φγ (3)
- 10. 4 Hund’s rule Hund’s rule is the manifestation of the same effect as we have seen in the parahelium - orthohelium effect ( the ”second” rule ) Hund’s first rule Full shells and subshells do have a total circular momentum of zero. This can be calculated and is allways valid. Hund’s second rule The total spin S should allways have the highest possible value. So as many of the single electron spins as possible should be parallel. The second rule appears more empirical and applies to a different magnetic quantum number of the electrons with parallel spins. If is of course not allowed to break the Pauli-principle. List of topics 10
- 11. 5 Example for the Hund’s rule Figure 3: Electron spins of the first elements. The elements carbon C, nitrogen N and oxygen O are those where the Hund’s rule have the biggest influence. We see electrons with parallel spins in the states m = −1, 0, +1, depending on the element. The magnetic quantum number m gives more or less the “direction” of the circular moment l. The s-states are the states where l = 0 and the p-states those where l = 1. The names for l = (2, 3, 4, ...) are (d, e, f, ...). The shell-name of the shell n = (1, 2, 3, 4, ...) is (K, L, M, N, ...). List of topics 11
- 12. In cases with more electrons do we get some exceptions. For example is the 4s subshell earlier filled than the 3d. This is caused by the smaller distance of the 4s to the core and thus by the lower energy, which is more stable. The mentioned rules work well for general considerations and for atoms with not to many electrons. 6 Number of states Usefull to know is the largest possible number of states for a given n which means until a special shell is filled. n−1 (2l + 1) = 2n2 . Nmax = 2 · (2) l=0 We get this formula by adding all the possible quantum number configurations: We get for each n every l in the range (0, 1, ..., n − 1), for each l every m in the range (−l, ..., −1, 0, 1, ..., l) and for each of these states 2 spin possibilities. List of topics 12
- 13. 7 Ionization energies Figure 4: Ionization energies. The shell properties would explain the structure in general - Periodic table and the Selfconsistent field List of topics 13
- 14. Especially at the first elements, we see the minimums at the elements with just one electron in the last shell and a maximum at the noble gas elements. The weak maxima are caused by filled subshells or by the maximum of parallel standing spins. After argon, it is more difficult to observe general tendences. The shell properties would explain the structure in general But there should be no closed shell at argon Details - Periodic table and the Selfconsistent field List of topics 14
- 15. 8 Hartree - Selfconsistent field Interaction energy of two charges depends on their distance|r − x|: W (|r − x|) = q1 q2 |r − x| The two charges are an electron and a little volume dV at x containing charge cloud of density ρ q1 → (−e) q2 → ρ(x)dV → ρ(x)d3 x ρ( x ) x The interaction energy of these two charges is dW (|r1 − r2 |) = 3 dV = d x x−r (−e)ρ(r2 ) 3 d r2 |r − x| r List of topics 15
- 16. Interaction with a cloud; summing over all the small volume elements - it means integrating over the whole volume of the cloud gives the potential energy W (r) = (−e)ρ(x) 3 d x |r − x| ρ( x ) x 3 dV = d x x−r r List of topics 16
- 17. If the charge cloud represents one electron in state ψi (x) and again integrating gives the potential energy due to the interaction with a (probability based density) cloud of electrons ρ(x) = (−e)|ψi (x)|2 If we have N electrons, each in its state, the total density becomes (−e)2 N |ψi (x)| ρ(x) = (−e) W (r) = 2 i=1 ρ( x ) x 3 dV = d x x−r r List of topics 17 N |ψi (x)|2 i=1 |r − x| d3 x
- 18. Now solving the Schr¨dinger equation with W (r), o (T + V + W ) ψi (x) = Ei ψi (x) We first need to know the W (r), but that depends on all the other N solutions N (−e)2 |ψi (x)|2 i=1 W (r) = |r − x| d3 x Approximation chain: First we choose some simple approximation, e.g. the hydrogenlike states, or we might know the states foranother atom. We call it (0) ψi (x) (0) From the set of all N ψi we construct e2 W (1) (r) = N i=1 (0) |ψi (x)|2 |r − x| d3 x In atomic units the whole Schr¨dinger equation is o − 1 2 2 − Z (1) (1) (1) + W (1) (r) ψi (x) = Ei ψi (x) r List of topics 18
- 19. (0) 1. step - choose arbitrary set of ψi N (0) → ψi (x) − 1 2 2 − (1) 2. step: take the set of ψi W (1) (r) = i=1 |r − x| from the 1. step (1) → ψi (x) 1 2 2 d3 x Z (1) (1) (1) + W (1) (r) ψi (x) = Ei ψi (x) r N − (0) |ψi (x)|2 − W (2) (r) = i=1 (1) |ψi (x)|2 |r − x| d3 x Z (2) (2) (2) + W (2) (r) ψi (x) = Ei ψi (x) r List of topics 19 (3)
- 20. (2) 3. step: take the set of ψi from the 2. step N (2) → ψi (x) − 1 2 2 − (3) 4. step: take the set of ψi W (3) (r) = i=1 |r − x| from the 3. step (3) → ψi (x) 1 2 2 d3 x Z (3) (3) (3) + W (3) (r) ψi (x) = Ei ψi (x) r N − (2) |ψi (x)|2 − W (4) (r) = i=1 (3) |ψi (x)|2 |r − x| d3 x Z (4) (4) (4) + W (4) (r) ψi (x) = Ei ψi (x) r List of topics 20
- 21. (4) 5. step: take the set of ψi from the 4. step N (4) → ψi (x) − 1 2 2 − (5) 6. step: take the set of ψi W (5) (r) = i=1 |r − x| from the 5. step (5) → ψi (x) 1 2 2 d3 x Z (5) (5) (5) + W (5) (r) ψi (x) = Ei ψi (x) r N − (4) |ψi (x)|2 − W (6) (r) = i=1 (5) |ψi (x)|2 |r − x| d3 x Z (6) (6) (6) + W (6) (r) ψi (x) = Ei ψi (x) r List of topics 21
- 22. Iteration chain N (n) ψi (x) → W (n+1) (r) = i=1 (n) |ψi (x)|2 |r − x| d3 x → (n+1) ψi (x) (n) This chain can continue, until the set of ψi produces a potential W (n+1) which (n) is the same as W (n) , which was the one to determine ψi . The potentials and functions become consistent, hence the name Selfconsistent field. Criterium for self-consistency: the (n+1)-th solution does not differ from th n-th solution N (n+1) |ψi (n) (x)|2 − |ψi (x)|2 d3 x < i=1 where ∝ 10−8 as a typical value. List of topics 22 (4)
- 23. 9 Ionization potentials of atoms !"##$% OOP O !"# O $%#& 1O 2 O 2"3)(.&+ OOOQA&RB O 1456 O '()& ) *(+,-./)%0-(+ 7189 O O ) ) ) :<5=> O =54> O ) A2&B *%+,: 7:89 O O ) ) ) ) >54: O A2&B *-+,: O ) ) D54 O A2&B *-+,: 7:E9 O ) : O 2& 4 O ?- O 2&;-/# O ?-0@-/# < O C& O C&)";;-/# =O C O C()(+ 6O * O *%)F(+ 115:6 O A2&B *-+,: *-.,: O ) GO $ O $-0)(.&+ 1<5=4 O A2&B *-+,: *-.,4 O ) DO H O HI".&+ 1456: O A2&B *-+,: *-.,< O ) >O J O J;/()-+& 1G5<: O A2&B *-+,: *-.,= O ) 1K O $& 11 O $% O $&(+ O !(3-/# :15=6 O =51< O A2&B A$&B *-+,: 7489 *-.,6 O O ) ) ) 1: O L. O L%.+&8-/# G56= O A$&B */+,: O ) ) 14 O M; O M;/#-+/# =5>> O A$&B */+,: 74E9 O ) 1< O !- O !-;-'(+ D51= O A$&B */+,: */.,: O ) 1= O N O N@(8E@()/8 1K5<> O A$&B */+,: */.,4 O ) 16 O ! O !/;,/) 1K546 O A$&B */+,: */.,< O ) 1G O *; O *@;()-+& 1:5>G O A$&B */+,: */.,= O ) 1D O M) O M).(+ 1=5G6 O A$&B */+,: */.,6 O ) List of topics 23
- 24. !"##$% !()*+ Q 9):9 Q ,-./ ,#$/ *+,-= ;95< *+.-+ Q Q ) ) ) Q ?4@A678 +)!! Q ,#$/ */,-= Q ) ) Q BA4'C678 +)(9 Q ,#$/ ;:C< */,-= Q ) */,-= !" Q #$ !0 Q 1 Q #$%&' Q 2&3455678 => Q ?4 =! Q BA == Q D6 Q D634'678 +)"= Q ,#$/ *+0-= Q ) =: Q E Q E4'4C678 +)*9 Q ,#$/ *+0-: */,-= Q ) =9 Q ?$ Q ?F$&8678 +)** Q ,#$/ *+0-( ;95< Q ) =( Q G' Q G4'%4'.5. *)99 Q ,#$/ *+0-( */,-= Q ) =+ Q H. Q I$&' *)"* Q ,#$/ *+0-+ */,-= Q ) =* Q ?& Q ?&J4@3 *)"+ Q ,#$/ *+0-* */,-= Q ) =" Q -6 Q -6AK.@ *)+9 Q ,#$/ *+0-" */,-= Q ) =0 Q ?7 Q ?&LL.$ *)*: Q ,#$/ *+0-!> ;95< Q ) :> Q M' Q M6'A 0):0 Q ,#$/ *+0-!> */,-= Q ) :! Q N4 Q N4@@678 +Q ,#$/ *+0-!> */,-= ;9L< Q := Q N. Q N.$84'678 *)0 Q ,#$/ *+0-!> */,-= */.-= Q :: Q #5 Q #$5.'6A 0)"! Q ,#$/ *+0-!> */,-= */.-: Q :9 Q B. Q B.@.'678 0)*( Q ,#$/ *+0-!> */,-= */.-9 Q :( Q O$ Q O$&86'. !!)"! Q ,#$/ *+0-!> */,-= */.-( Q :+ Q 1$ Q 1$PL3&' !9 Q ,#$/ *+0-!> */,-= */.-+ Q List of topics 24
- 25. !" W #$ !/ W 01 *+ W +6*7 W ,-$. ,#$. *+,-*? 89:; *./-C W *.0-" ) W ) !7 W <$ W <'$()'325 96/ W ,#$. *1/-C W ) ) != W > W >''$325 "6!7 W ,#$. 8+4; *1/-C W ) +? W @$ W @3$A()325 "67+ W ,#$. *.,-C *1/-C W ) +* W B1 W B3(1325 "677 W ,#$. *.,-+ 89:; W ) +C W D( W D(E%14F)25 /6* W ,#$. *.,-9 89:; W ) +! W GA W GFAH)F'325 /6C7 W ,#$. *.,-9 *1/-C W ) ++ W 02 W 02'HF)325 /6!/ W ,#$. *.,-/ 89:; W ) +9 W 0H W 0H(4325 /6+" W ,#$. *.,-7 89:; W ) +" W I4 W IJEEJ4325 76!+ W ,#$. *.,-*? W ) ) +/ W -K W <3ELF$ /697 W ,#$. *.,-*? 89:; W ) +7 W M4 W MJ45325 76== W ,#$. *.,-*? *1/-C W ) += W N) W N)4325 96/= W ,#$. *.,-*? *1/-C 89&; W 9? W <) W G3) /6!+ W ,#$. *.,-*? *1/-C *10-C W 9* W <1 W -)'35()% 76"+ W ,#$. *.,-*? *1/-C *10-! W 9C W GF W GFEE2$325 =6?* W ,#$. *.,-*? *1/-C *10-+ W 9! W N W N(43)F *?6+9 W ,#$. *.,-*? *1/-C *10-9 W 9+ W OF 99 W M: W OF)() W MF:325 *C6*! W !67= W ,#$. ,OF. *.,-*? 8":; *1/-C W *10-" ) W ) 9" W PJ W PJ$325 96C* W ,OF. *2/-C W ) ) 9/ W QJ W QJ)'HJ)25 9697 W ,OF. 894; *2/-C W ) 97 W MF W MF$325 969+ W ,OF. 8+R; 894; *2/-C W 9= W I$ List of topics W #$%&'() W 02134325 W I$J:F(4%5325 96+" W ,OF. *.3-! *2/-C W ) "? W B4 W BF(4%5325 969! W ,OF. *.3-+ *2/-C W ) "* W I5 W I$(5F'H325 9699 W ,OF. *.3-9 *2/-C W ) "C W <5 W <J5J$325 96"+ W ,OF. *.3-" *2/-C W ) "! W S2 W S2$(&325 96"/ W ,OF. 25 *.3-/ *2/-C W )
- 26. Screened potential and Centrifugal barrier 0.5 Coulomb potential L=2 L=1 0 L=0 −0.5 0 1 2 3 4 5 6 7 8 6 7 8 0.5 Hartree potential L=2 L=1 0 L=0 −0.5 0 − 1 Z r + 2 3 L(L + 1) r2 4 5 vs. − 26 Z − 1 −αr 1 e − r r List of topics + L(L + 1) r2 (5)
- 28. Figure 5: Selfconsistent field calculations compared to coulomb - real results Here we note the position of the turning point - same for both Coulomb and SCF case. This is true only for the 1s states. List of topics 28
- 29. Figure 6: Hartree Atomic potential This figure shows results for oxygen with the potential and energies obtained from a calculation with SCF program List of topics 29
- 30. results for oxygen with the potential and energies obtained from a calculation with SCF program compared with hydrogen-like levels List of topics 30
- 31. 10 Expectation value of energy for N-particles Hamiltonian for N-particles - the repulsion - we sum over pairs of coordinates N 2 2 e Ze 1 2 + H (r1 , r2 , ...rN ) = − ri − 2 ri |ri − rj | i=1 (i,j)pairs Without antisymmetrization - independent particles - product function only 1 term -each particle has its orbital ψa ,ψb ,ψc .....ψn Φ (r1 , r2 , ...rN ) → ψa (r1 )ψb (r2 )ψc (r3 )......ψn (rN ) With antisymmetrization - the Slater determinant 1 ΦHF (r1 , r2 , ...rN ) → √ a,b,...n N! List of topics 31 ψa (r1 )ψb (r1 )......ψn (r1 ) ψa (r2 )ψb (r2 )......ψn (r2 ) ....... ψa (rN )ψb (rN )......ψn (rN )
- 32. For N-particles - the repulsion - we sum over pairs of coordinates N 2 2 1 2 Ze e Φ (r1 , r2 , ...rN ) = EΦ (r1 , r2 , ...rN ) − + ri − 2 ri |ri − rj | i=1 (i,j)pairs Expectation value with the independent particles - product function - only 1 term Φ (r1 , r2 , ...rN ) → ψa (r1 )ψb (r2 )ψc (r3 )......ψn (rN ) becomes n E = Φ| H |Φ → ψα | T − α=a Ze2 |ψα + r ψβ ψα | (α,β)pairs e2 |ψβ ψα |r − r | The sum over coordinate pairs becomes sum over pairs of orbitals This remains true for Slater determinants - but it must be shown. There are N ! terms in the Slater determinant, the left and right give (N !)2 terms, and there are N (N − 1)/2 coordinate pairs. Thus (N !)2 N (N − 1)/2 terms. Due to the normalization, this becomes only N (N − 1) terms, N (N − 1)/2 direct terms and N (N − 1)/2 exchange terms List of topics 32
- 33. It should be shown that when the Slater determinant is used ψa (r1 )ψb (r1 )......ψn (r1 ) 1 ψa (r2 )ψb (r2 )......ψn (r2 ) ΦHF (r1 , r2 , ...rN ) → √ a,b,...n N ! ....... ψa (rN )ψb (rN )......ψn (rN ) the expectation value will give (final result shown here first) n ΦHF H ΦHF = ψα | T − j=α Ze2 |ψα r ψβ ψα | e2 |ψβ ψα |r − r | ψβ ψα | + e2 |ψα ψβ |r − r | (α,β)pairs − (α,β)pairs (6) The last term - exchange energy. On the previous page product function (1 term) is shown - no exchange energy Next 3 slides: 1. Helium, 2. Lithium examples, 3. Counting nonzero terms List of topics 33
- 34. r 12 −e2 r1 r2 For Helium − h2 ¯ 2me 2 r1 − −e1 +Ze Z e2 h2 ¯ − r1 2me 1 ΦHF (r1 , r2 ) = √ a,b 2 2 r2 − Z e2 e2 Ψ (r1 , r2 ) = E Ψ (r1 , r2 ) + r2 |r1 − r2 | 1 = √ [ψa (r1 )ψb (r2 ) − ψb (r1 )ψa (r2 )] 2 ψa (r1 ) ψb (r1 ) ψa (r2 ) ψb (r2 ) and the energy becomes ΦHF H ΦHF = + Ze2 Ze2 |ψa + ψb | T − |ψb r r e2 e2 ψa ψb | |ψa ψb − ψa ψb | |ψb ψa |r − r | |r − r | ψa | T − The last term - exchange energy - and there is only one pair. List of topics Lithium example 34 (7)
- 35. −e3 r 13 r 23 r3 r 12 −e 2 −e1 r1 r2 Lithium +Ze − h ¯2 2me + 2 r1 − Z e2 h ¯2 − r1 2me 2 r2 − 6 × 6 × 3-pair terms Z e2 h ¯2 − r2 2me 2 r3 − Z e2 r3 e2 e2 e2 + + Ψ (r1 , r2 , r3 ) = E Ψ (r1 , r2 , r3 ) |r1 − r2 | |r1 − r3 | |r2 − r3 | (8) 1 √ { ψα (1) ψβ (2) ψγ (3) + 3! − ψγ (1) ψβ (2) ψα (3) List of topics ψβ (1) ψγ (2) ψα (3) + − ψα (1) ψγ (2) ψβ (3) Helium example ψγ (1) ψα (2) ψβ (3) − ψβ (1) ψα (2) ψγ (3)} Next slide: Counting nonzero terms 35
- 36. 1 3! a b c + a b c + a b c + a b c − a b c − a b c − a + + + + + b c + + c a + e2 r2 3 e2 r2 3 e2 r2 3 e2 r2 3 e2 r2 3 e2 r2 3 b + b c a + − b a − c − c b a − − a c − b a b c a a b c c a b a c b c e2 r2 3 e2 r2 3 b c a a b b c e2 r2 3 c a b a c a b b c e2 r2 3 a c c b a a c b c e2 r2 3 b a a c a a b c e2 r2 3 c b + + + − − − − b b c a b Evaluation of the first six terms of the total 36 terms. For each further group of 6 a very similar procedure would follow. From each six, two remain, totaling 12 nonzero terms List of topics 36
- 37. 11 N particles in Slater determinant - Total Energy Summary N Φ| H |Φ = j=1 Ze2 |ψj + ψj | T − r (i,j)pairs e2 ψj ψi | |ψj ψi |r − r | 2 − ψj ψi | (i,j)pairs e |ψi ψj |r − r | (9) We will use also a more compact notation, instead of orbitals ψi we use orbitals |α , i.e. the index becomes the name of the function Φ| H |Φ = α|(T + V )|α + α [ αβ|Vee |αβ − αβ|Vee |βα ] (10) pairs αβ List of topics 37
- 38. Schr¨dinger equation from variational principle: minimize o Φ| H |Φ Φ|Φ (≥ Eg.s. ) which can be written as minimalization with constraint Φ | Φ = 1 ; minimize Φ| H |Φ − λ Φ | Φ Φ |Φ −1=0 λ Lagrange multiplier Derivative - differential f (x) → df (x) dx → df = df (x) dx dx Functional derivative - functional F(f ); variation of the functions f (x), δf F(f ) → δF(f ) δf → δF = δF(f ) δf δf (11) Variation of Φ| H |Φ is taken (considering the complex nature of |Φ ) as δ ( Φ| H |Φ ) = δΦ| H |Φ With arbitrary variations |δΦ δΦ| H |Φ − λ δΦ | Φ −→ H |Φ − λ |Φ = 0 −→ H |Φ Thus the Lagrange multiplier plays the role of energy eigenvalue 38 = λ |Φ List of topics
- 39. Deriving Hartree-Fock: For N electrons this energy must be minimized Φ| H |Φ = [ αβ|Vee |αβ − αβ|Vee |βα ] α|(T + V )|α + α (12) pairs αβ with respect to the N orbitals |α , |β , etc. , with N conditions α|α = 1, β|β = 1, i.e. with N Lagrange multipliers εα , εβ ....... δΦ(α → δα)| H |Φ − εα δα | α = 0 Which results in N equations (one for each of the N orbitals) [ (δα)β|Vee |αβ − (δα)β|Vee |βα ] − εα δα | α δα|(T + V )|α + = 0 (13) β Leaving for a while out the exchange-related term, we can as in prev. slide reduce this by removing δα| [ β|Vee |β ] |α − εα | α (T + V )|α + =0 β which is exactly the Hartree method. The exchange term leads to complications → List of topics 39
- 40. Reducing the full equation [ (δα)β|Vee |αβ − (δα)β|Vee |βα ] − εα δα | α δα|(T + V )|α + = 0 (14) β we get the Hartree Fock Equations (N orbitals) [ β|Vee |β ] |α − (T + V )|α + β [ β|Vee |α ] |β − εα | α =0 β The complexity of the exchange term becomes clear when we write it explicitely with coordinates and integrations. Hartree Fock Equations: The direct term and the exchange term: WHF = W d − W ex occ ∗ ψb (x) WHF ψa (r) = b e2 ψb (x)d3 x ψa (r)− |r − x| occ ∗ ψb (r) b ψb (x) e2 ψa (x)d3 x |r − x| where the exchange potential is nonlocal W d ψa → W d (r)ψa (r) W ex ψa (r) → List of topics 40 W ex (r, x)ψa (x)d3 x
- 41. Hartree-Fock total energy and sum of orbital energies with a shorthand notation ϕα (r) → |ϕα → |α Slater determinant Φα,β,....,ν → |α, β, ...., ν We have started our work with the Hartree-Fock equations by evaluating Φα,β,....,ν | T +V + pairs ij e2 |ri − rj | |Φα,β,....,ν (15) This we have evaluated as Φ| H |Φ = α|(T + V )|α + α [ αβ|Vee |αβ − αβ|Vee |βα ] (16) pairs αβ From this expression we have obtained Hartree-Fock equations by the variational procedure. List of topics 41
- 42. The Hartree-Fock equation can be then written as β| T +V + β e2 |β |r − r | |α − β| β e2 |α |r − r | |β = εα |α (17) We form the matrix element with the given α| α| T + V + β| β e2 |β |r − r | |α − α| β| β e2 |α |r − r | |β = εα (18) since α|α = 1. This can be rewritten as εα = α|(T + V )|α + [ βα|Vee |βα − βα|Vee |αβ ] (19) β=α And now we can explore what is the sum of all εα εα = α|(T + V )|α + α α [ βα|Vee |βα − βα|Vee |αβ ] (20) α β=α which we can compare with the above Φ| H |Φ Φ| H |Φ = α|(T + V )|α + α [ αβ|Vee |αβ − αβ|Vee |βα ] (21) pairs αβ 42
- 43. The two expressions are very similar, but they differ in fact by all the interaction term, since it is counted twice in the sum: Fαβ = 2 α β=α Fαβ pairs αβ for any set of objects that are symmetric Fαβ = Fβα Our objects are symmetric, because they are in fact of the type Hαβ,αβ . The wavefunctions are antisymmetric, |αβ = −|βα . Thus, surprisingly perhaps Φ| H |Φ = εα α but as we derived here Φ| H |Φ List of topics = εα − α [ αβ|Vee |αβ − αβ|Vee |βα ] pairs αβ 43 (22)
- 44. Evaluation of the repulsion term using the multipole expansion 1 = |r1 − r2 | LM L r< 4π YLM (ˆ1 ) YLM (ˆ2 ) r r L+1 2L + 1 r> (23) where r< = r1 , r> = r2 for |r1 | < |r2 | r< = r2 , r> = r1 for |r1 | > |r2 | Evaluation of the matrix element in general case d3 r1 d3 r2 ψn1 l1 m1 (r1 ) ψn2 l2 m2 (r2 ) 1 ψn l m (r1 ) ψn2 l2 m2 (r2 ) (24) |r1 − r2 | 1 1 1 is performed separately over the radial and angular parts 2 r1 dr1 dˆ1 r 2 r2 dr2 dˆ2 r 1 |r1 − r2 | r r Rn1 l1 (r1 )Yl1 m1 (ˆ1 ) Rn2 l2 (r2 )Yl2 m2 (ˆ2 ) Rn1 l1 (r1 )Yl1 m1 (ˆ1 ) Rn2 l2 (r2 )Yl2 m2 (ˆ2 )(25) r r where dˆi means the integration over dΩi = sin θi dθi dϕi . List of topics r 44
- 45. The evaluation of general case - angular integrals of three Ylm ’s CL = Yli mi (θ, ϕ)YLM (θ, ϕ)Yli mi (θ, ϕ)dΩ (26) For the case of both s-states, li = 0 mi = 0 only L = 0 M = 0 are nonzero; The sum reduces to one term. The angular factors give value one, since the (YL=0M =0 )2 = (4π)−1 cancels the corresponding factor in the multipole expansion and due to the normalization. List of topics 45
- 46. 12 Configuration mixing Consider the usual: Hx (x)ϕα (x) = Eα ϕα (x) Hy (y)χβ (y) = Eβ χβ (y) For any Φ(x) Φ(x) = cα ϕα (x) Ξ(y) = dβ χβ (y) For any Ξ(x) Take now a general Ψ(x, y). First look at y as a parameter, Ψ(x, y0 ) Ψ(x, y0 ) → Φ(x) = cα (y0 )ϕα (x) for every y0 ; Thus we get a new function of y; cα (y) = dβ (α)χβ (y) Inserting back: Ψ(x, y) = dβ (α)χβ (y)ϕα (x) 46
- 47. Or, with a simpler notation Ψ(x, y) = dβα χβ (y)ϕα (x) In the case of Helium, for example, the H(x) and H(y) are identical and so are the χβ (y) and ϕα (x). This becomes configuration mixing. Ψ(x, y) = dβα ϕβ (y)ϕα (x) The coefficients are found by diagonalization. For three coordinate sets - e.g. for Lithium : Ψ(x, y, z) = Dγβα ϕ(z)ϕβ (y)ϕα (x) List of topics 47
- 48. END OF THE PRESENTATION List of topics 48
