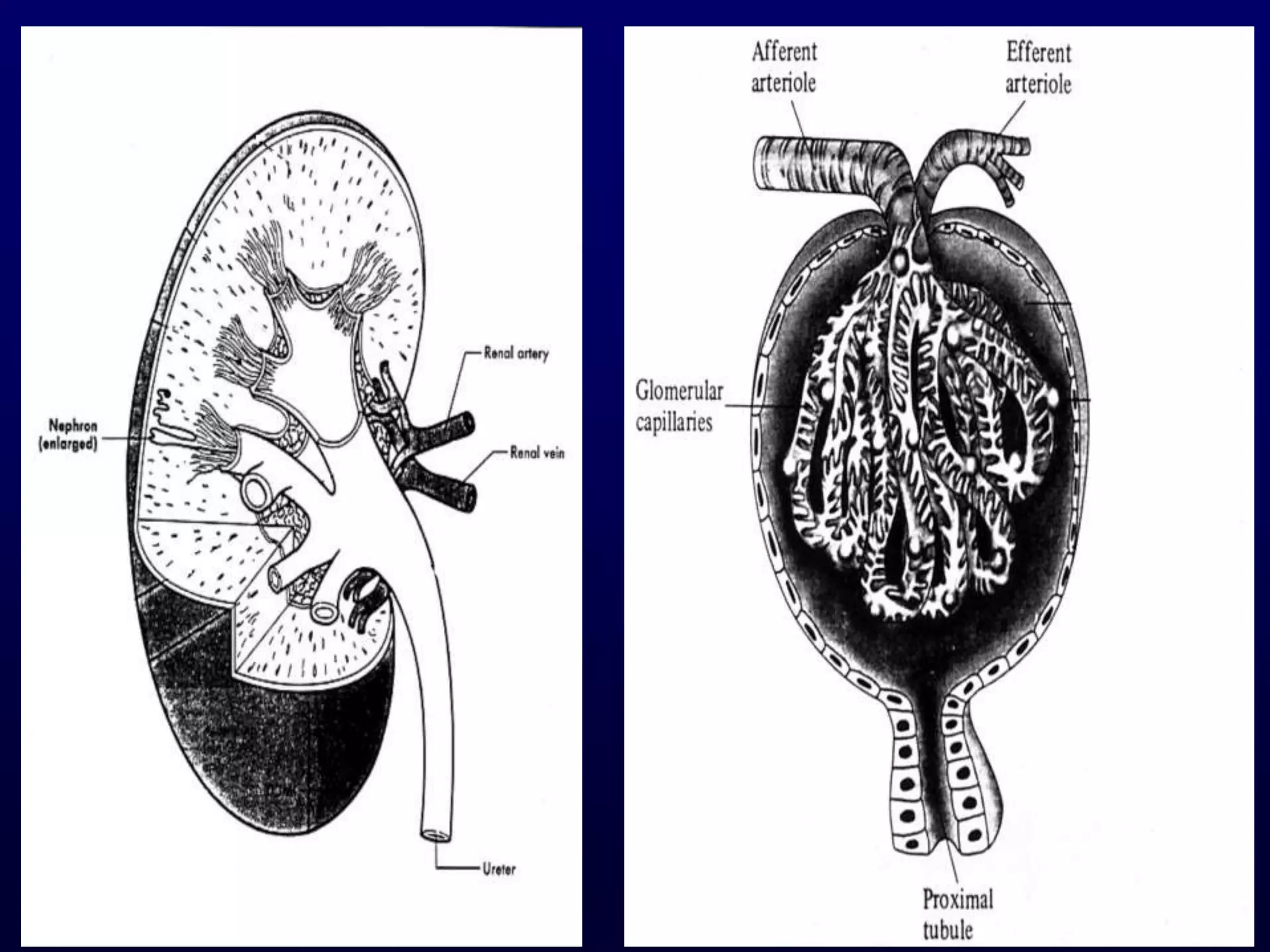
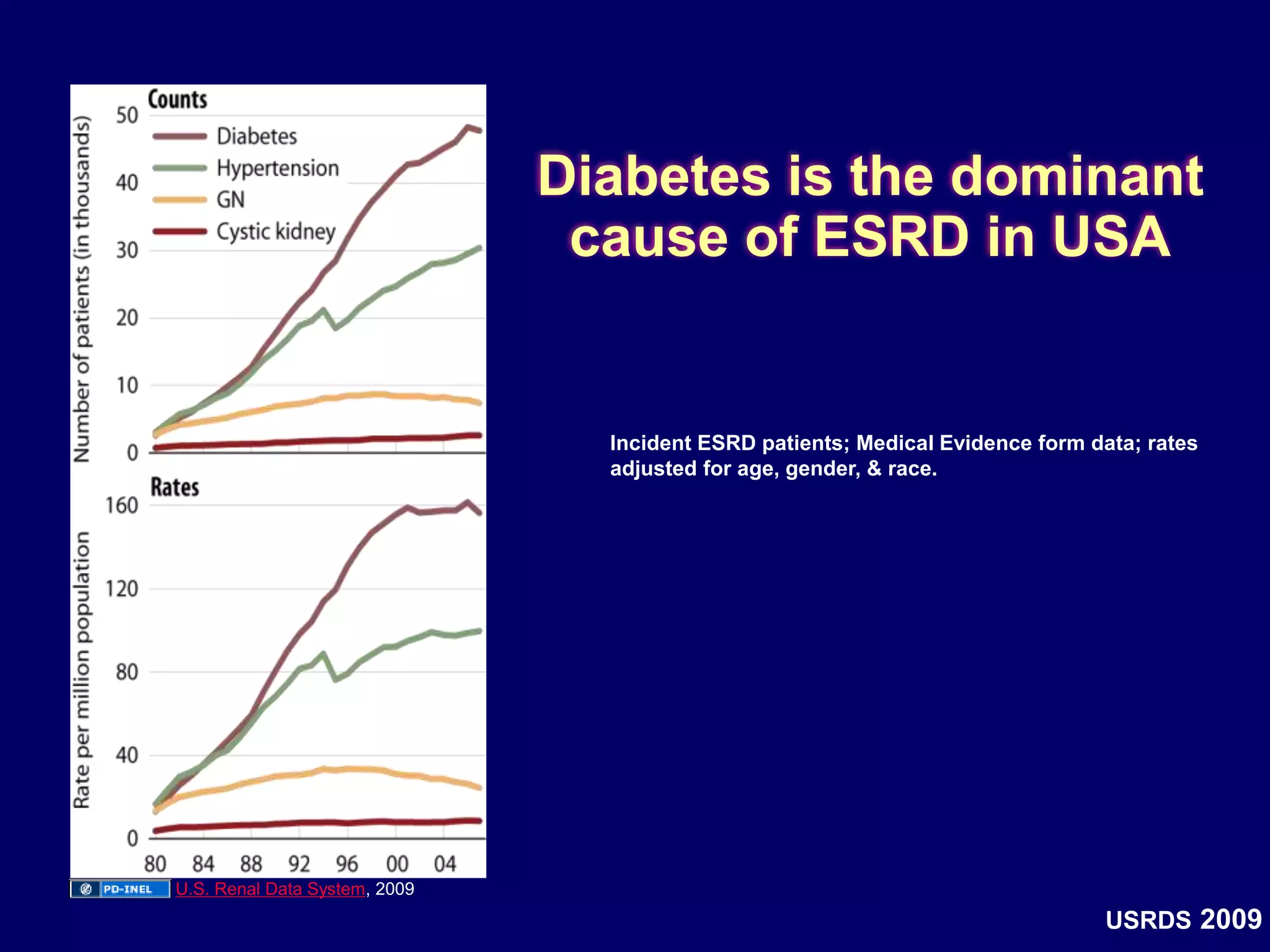
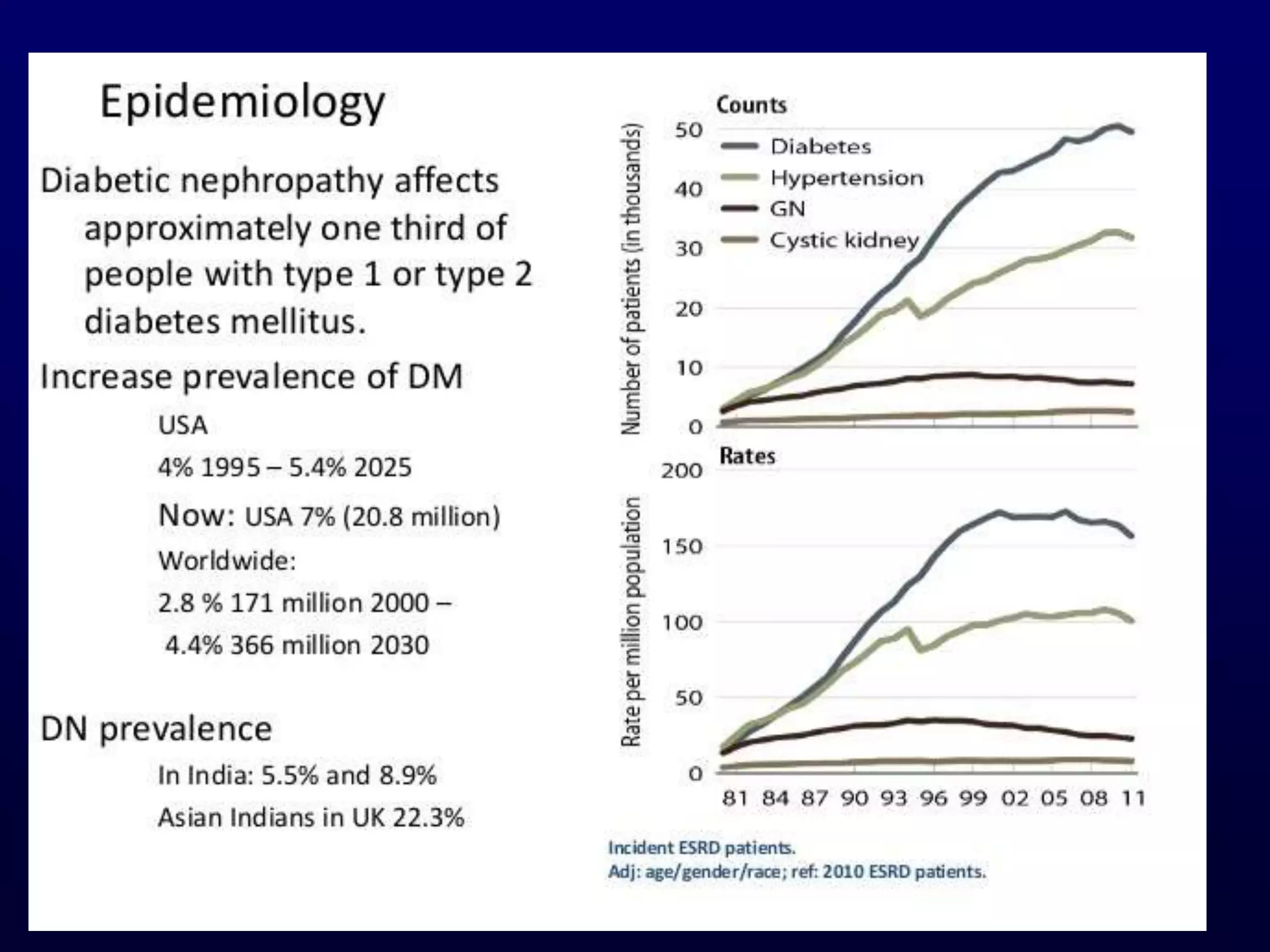
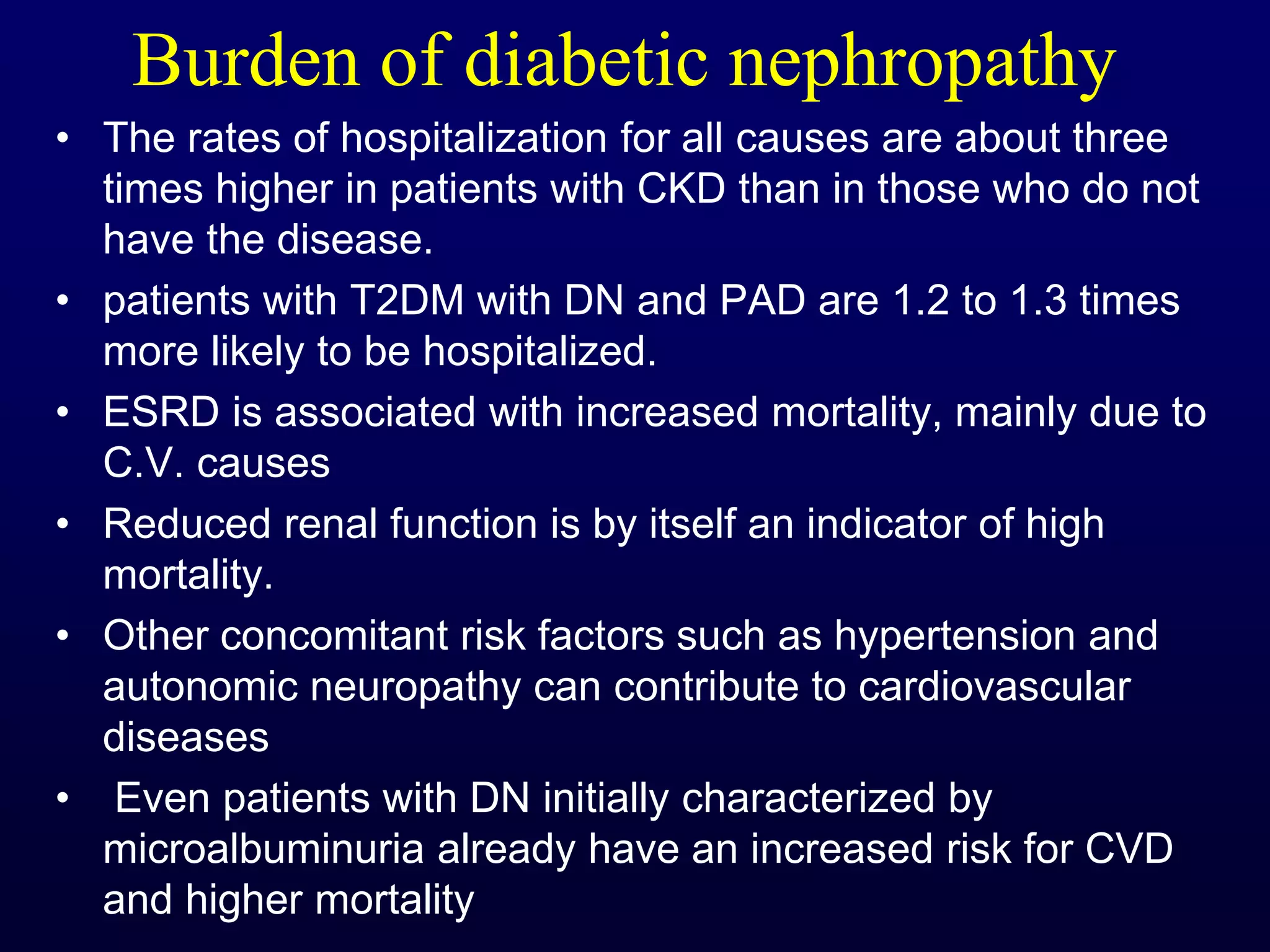
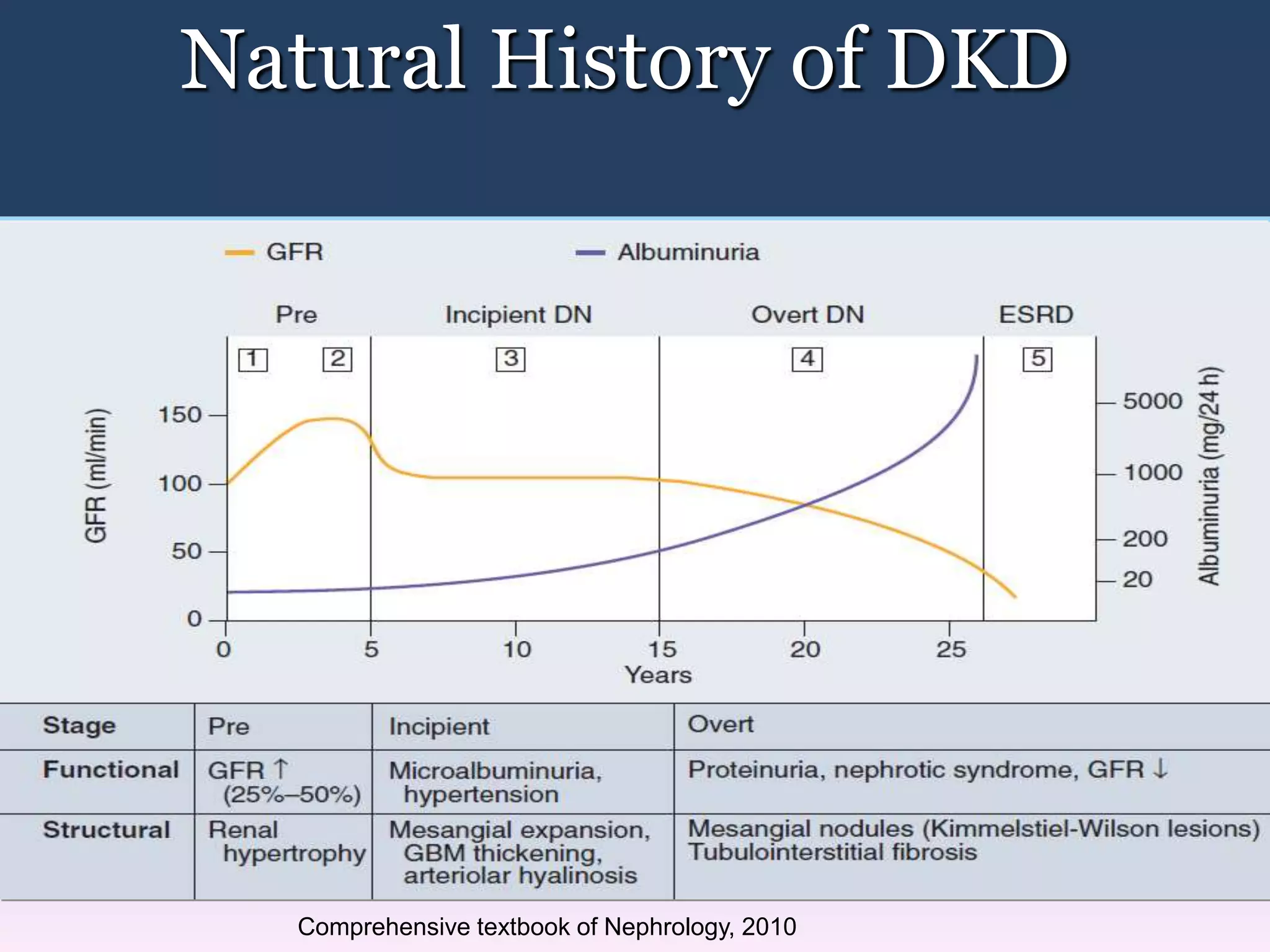
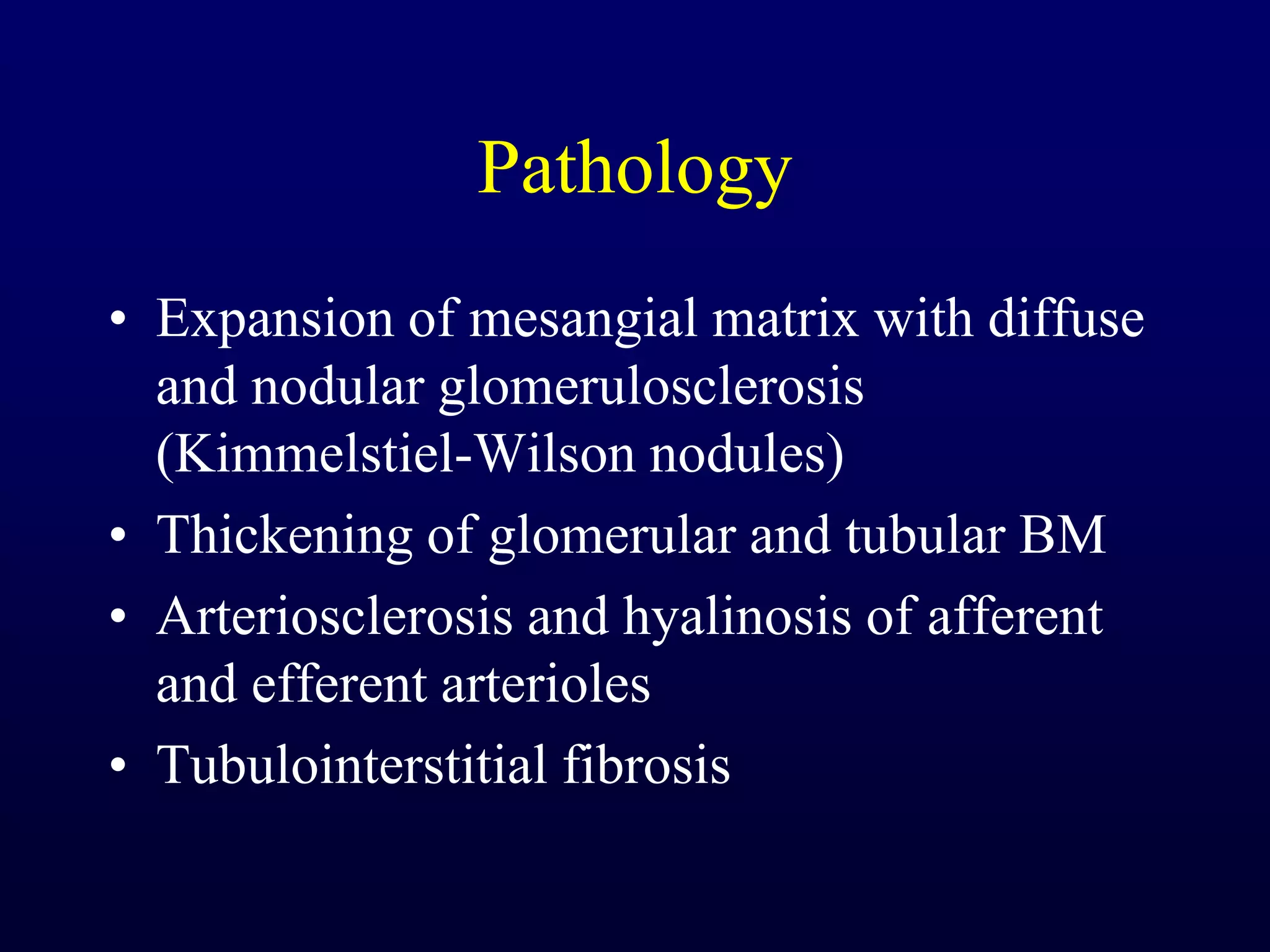
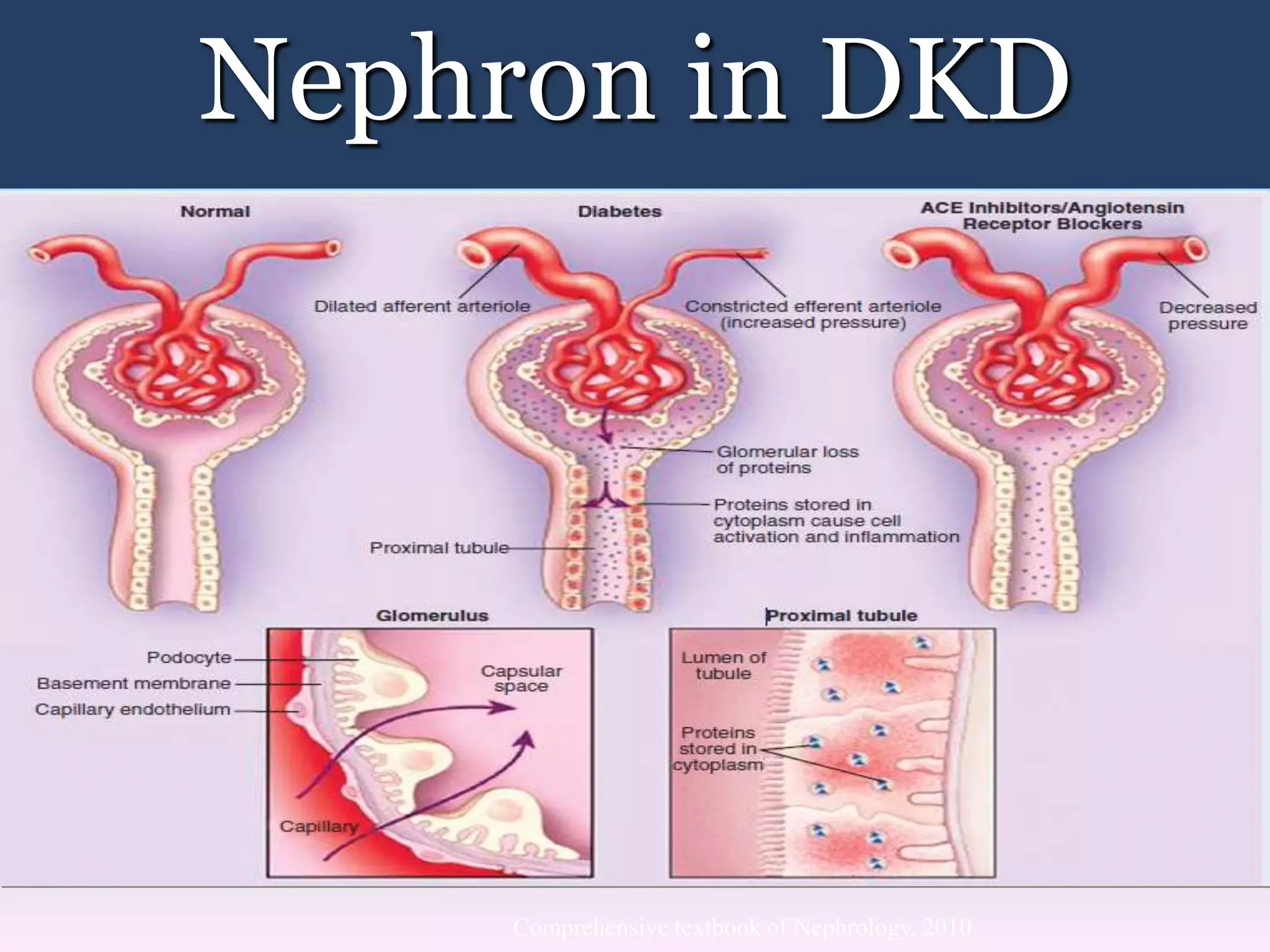
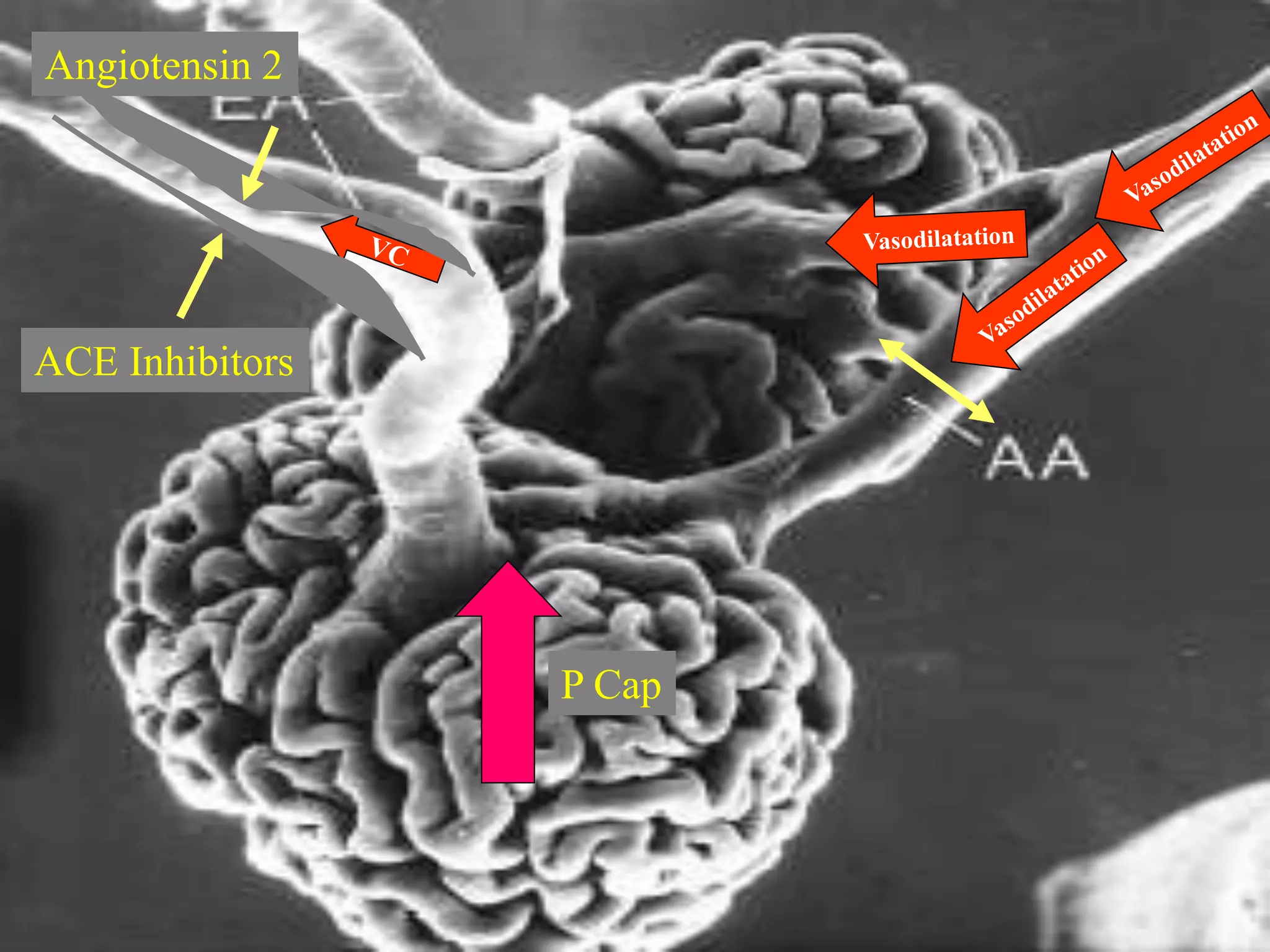
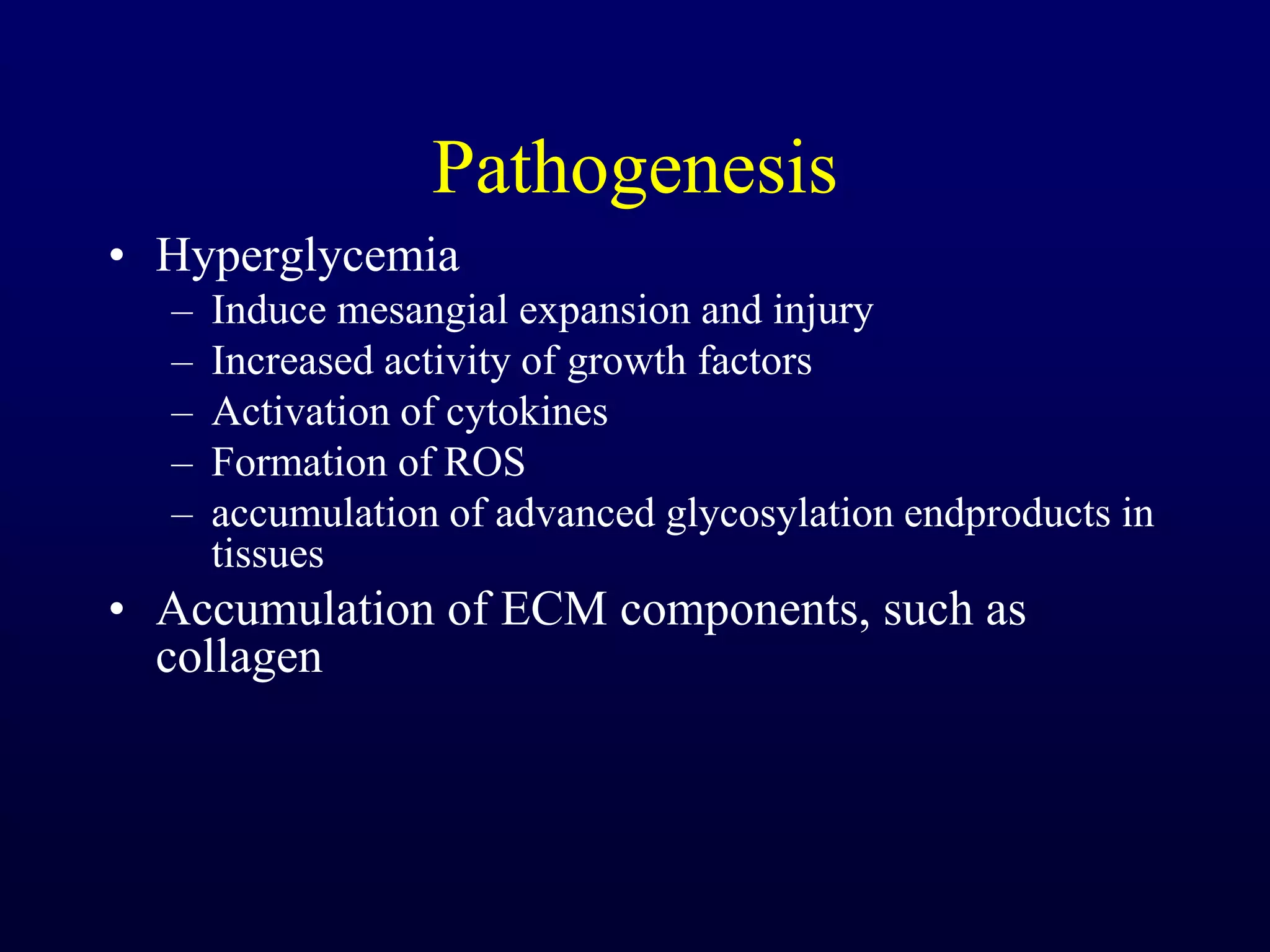
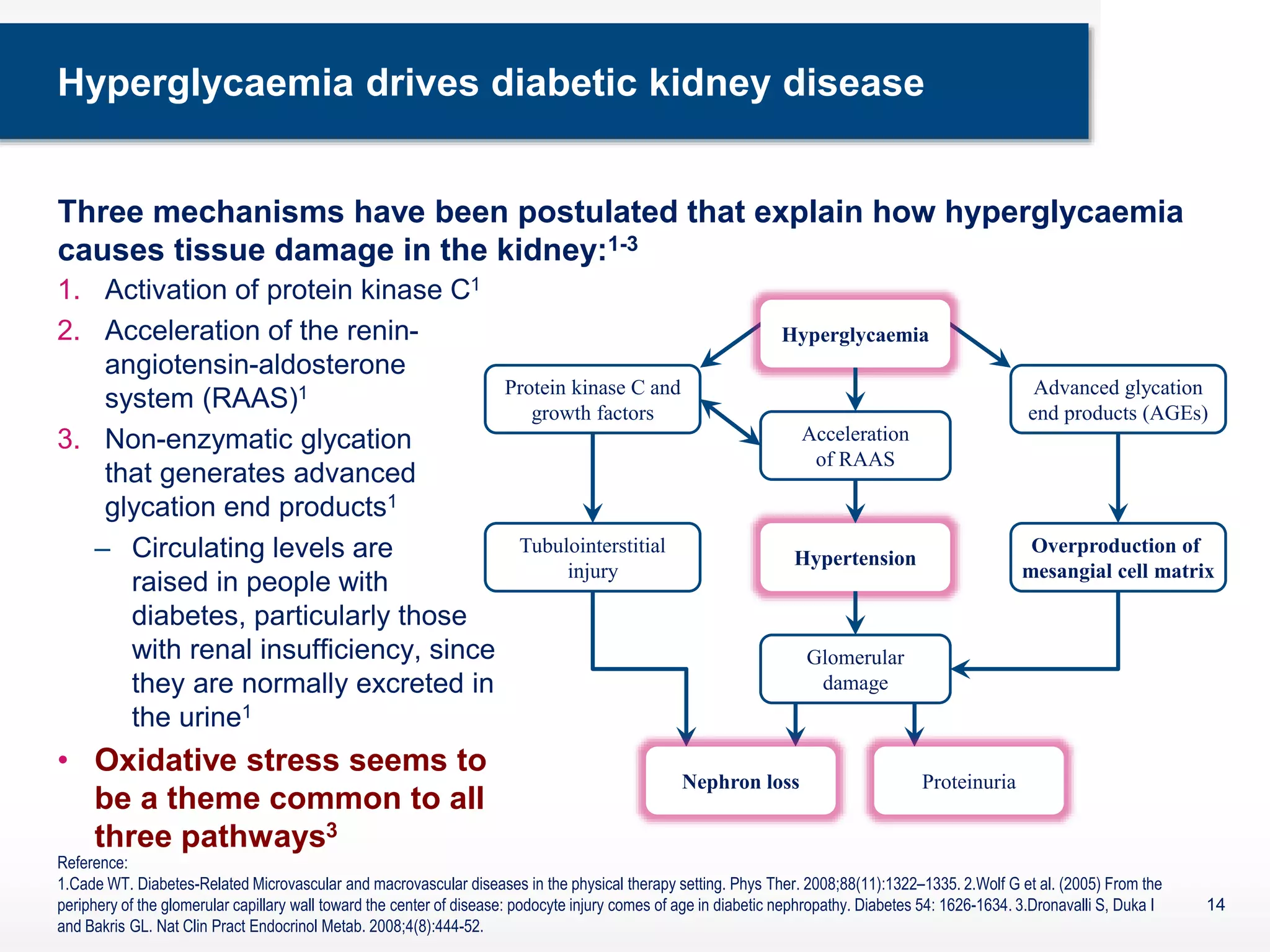
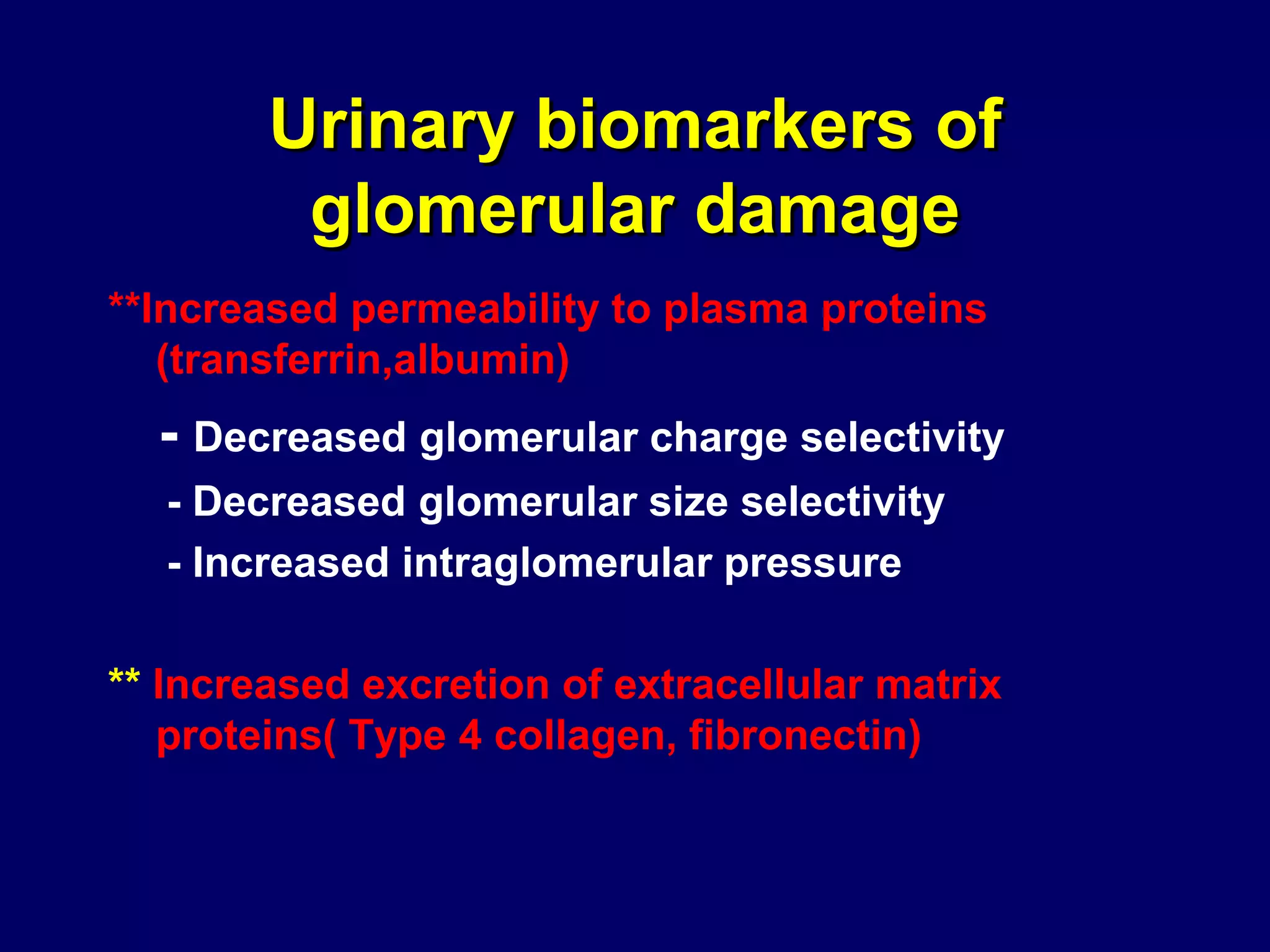
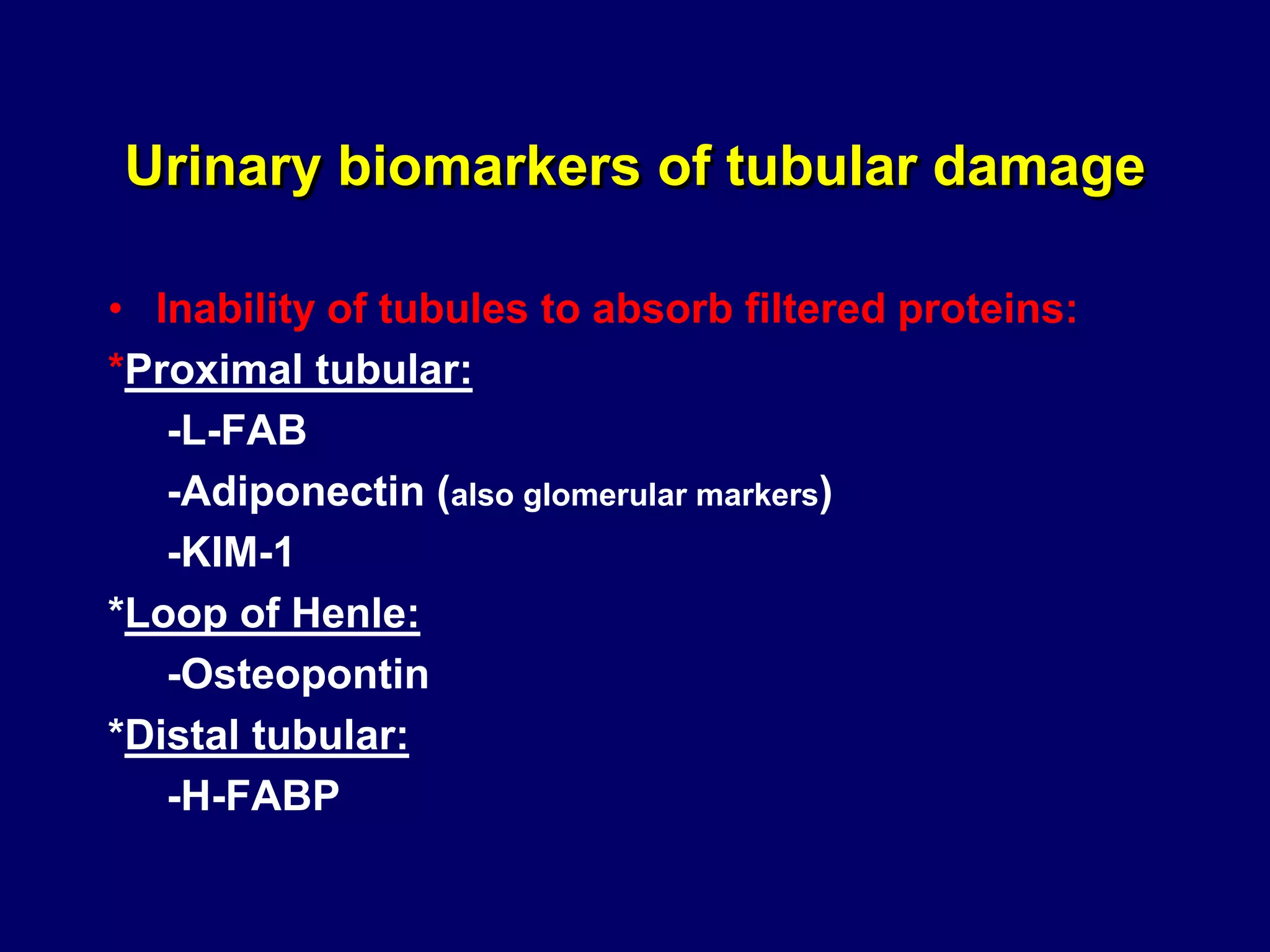
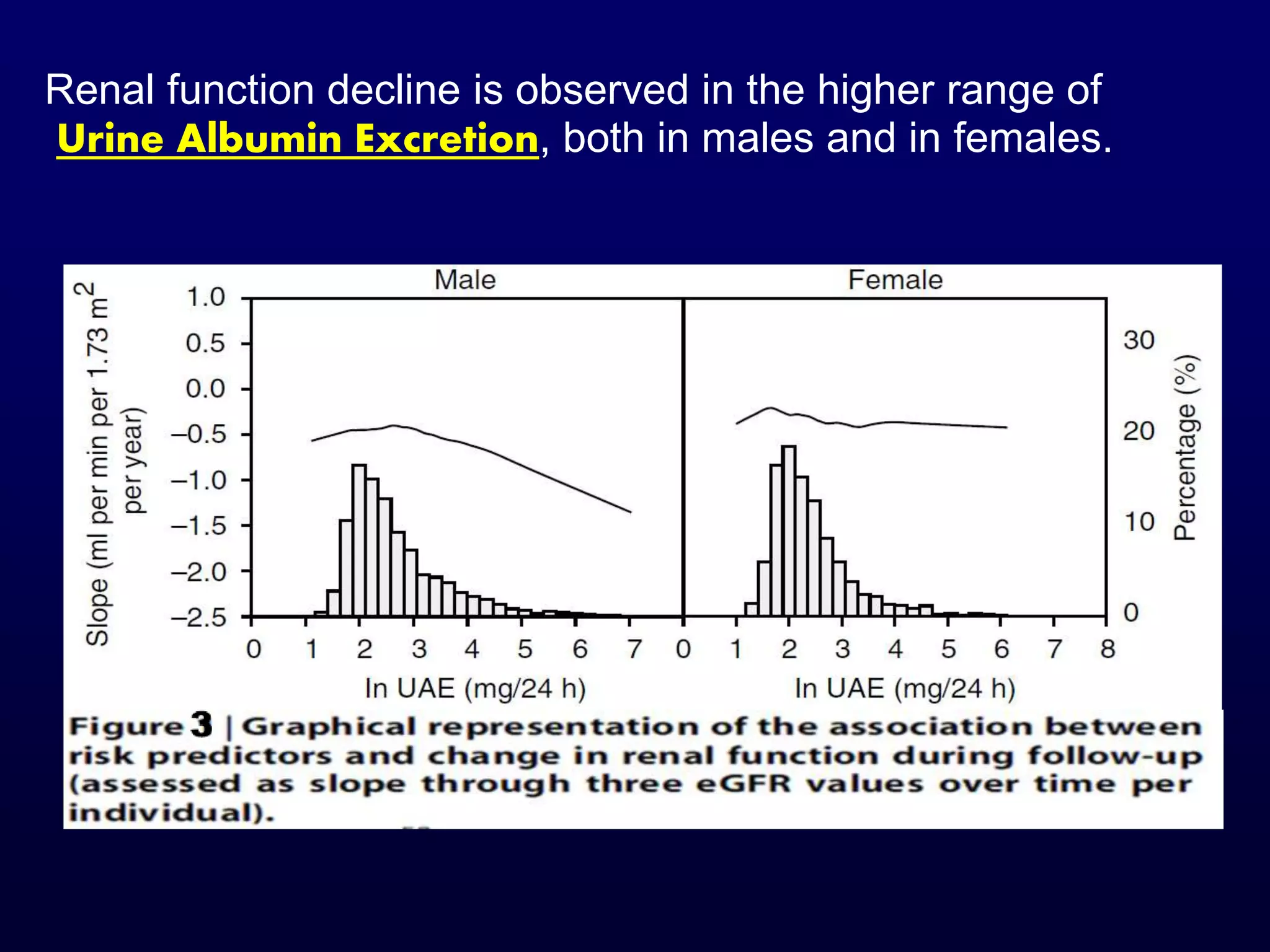
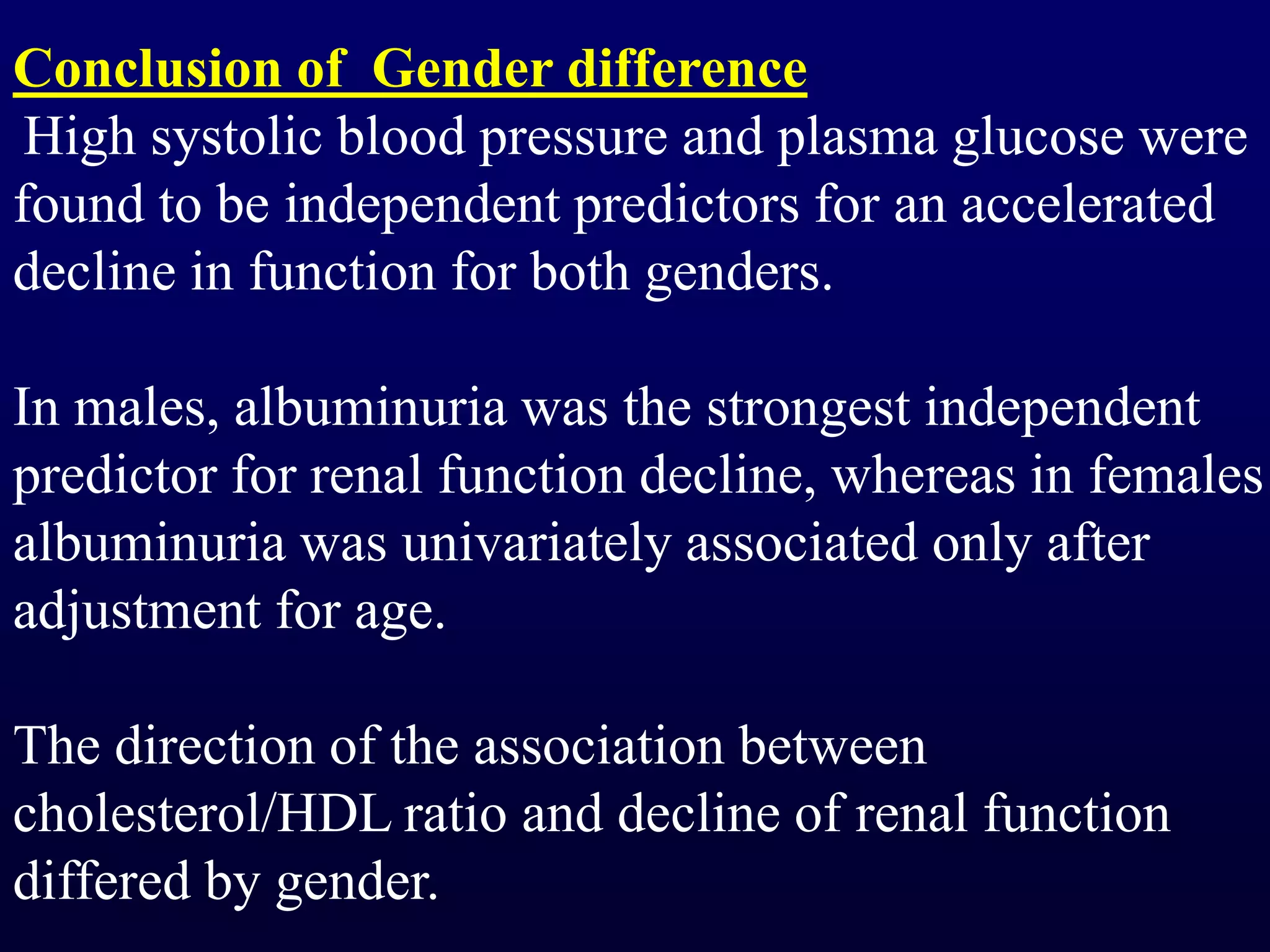
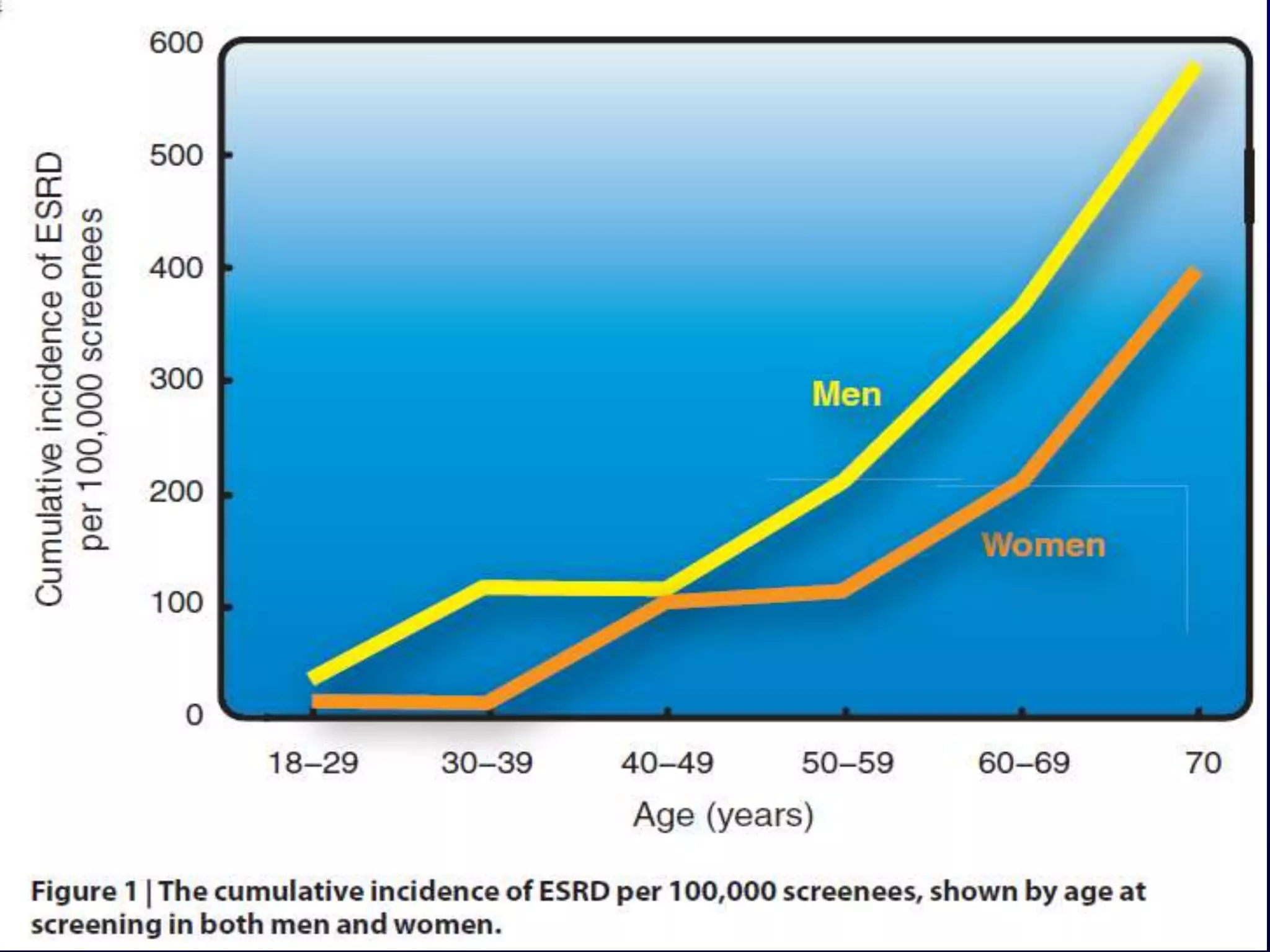
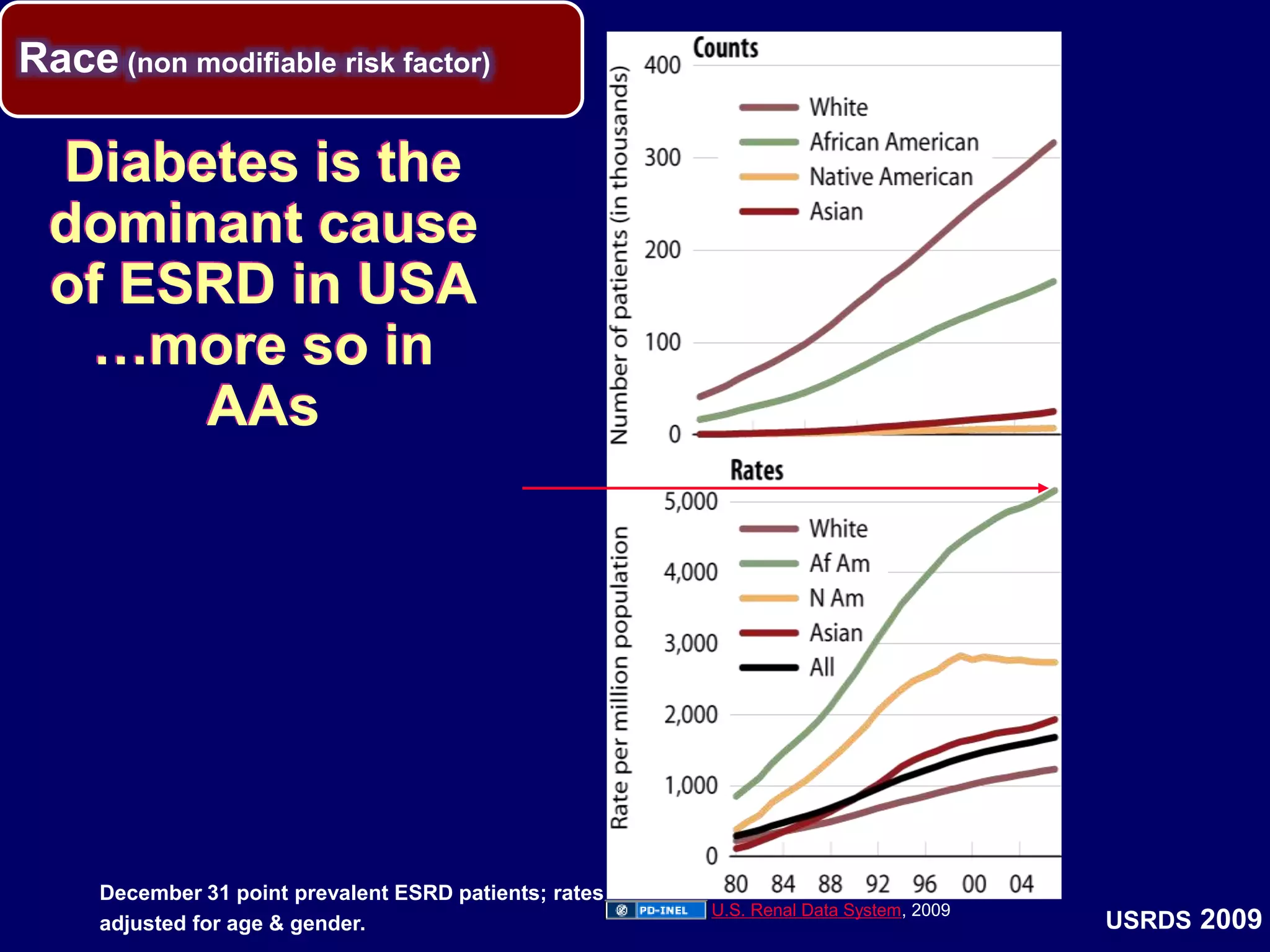
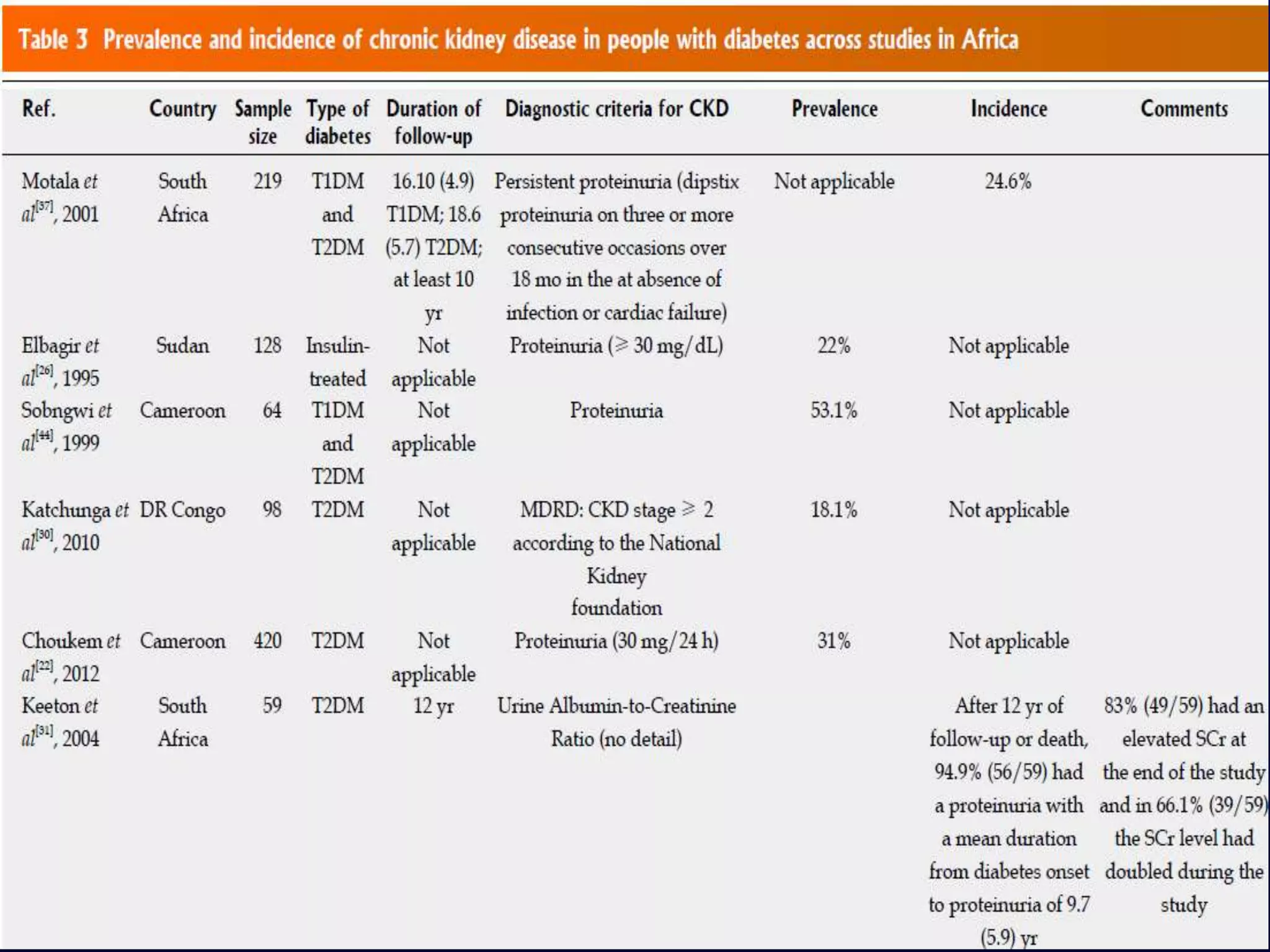
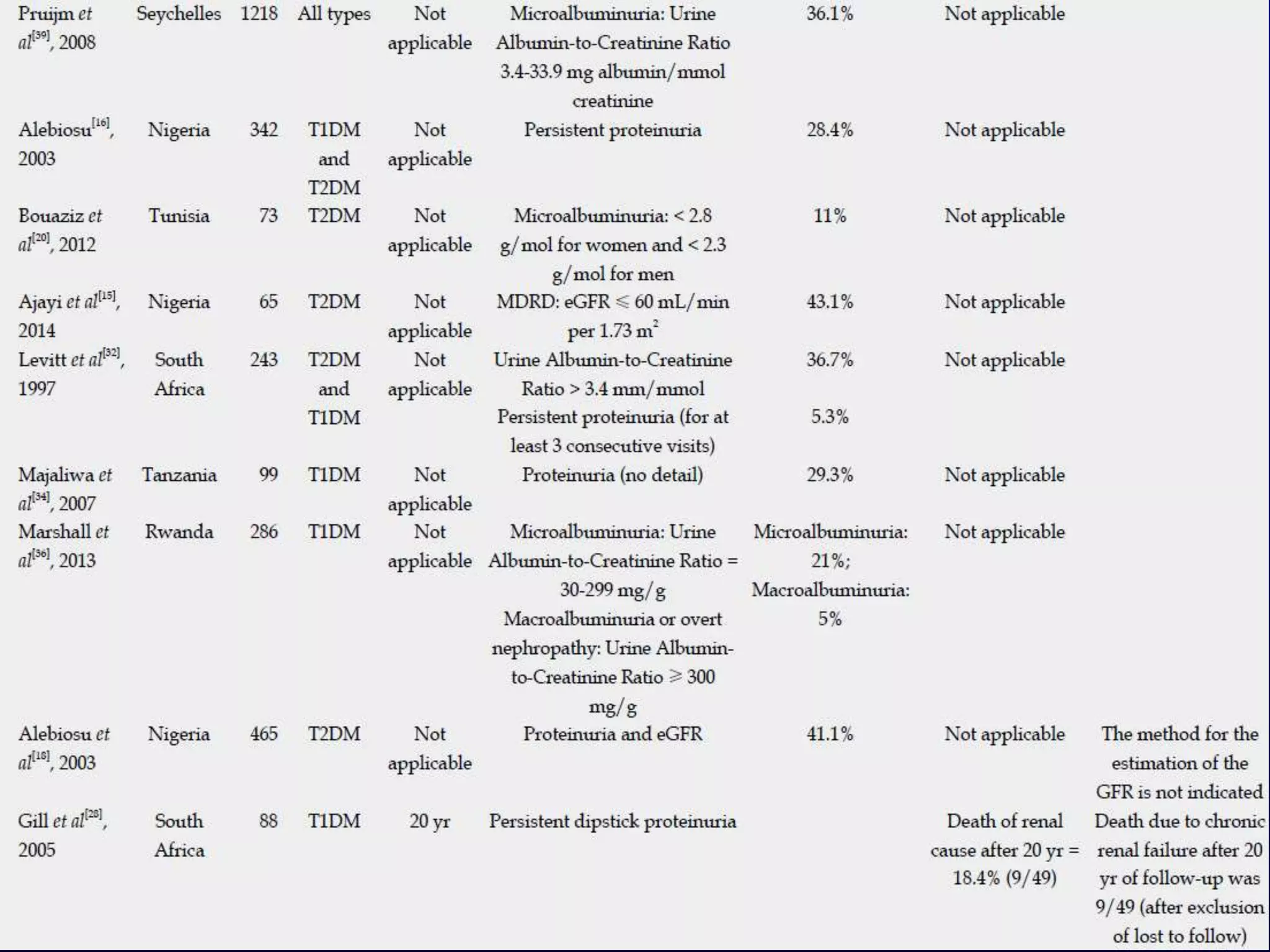
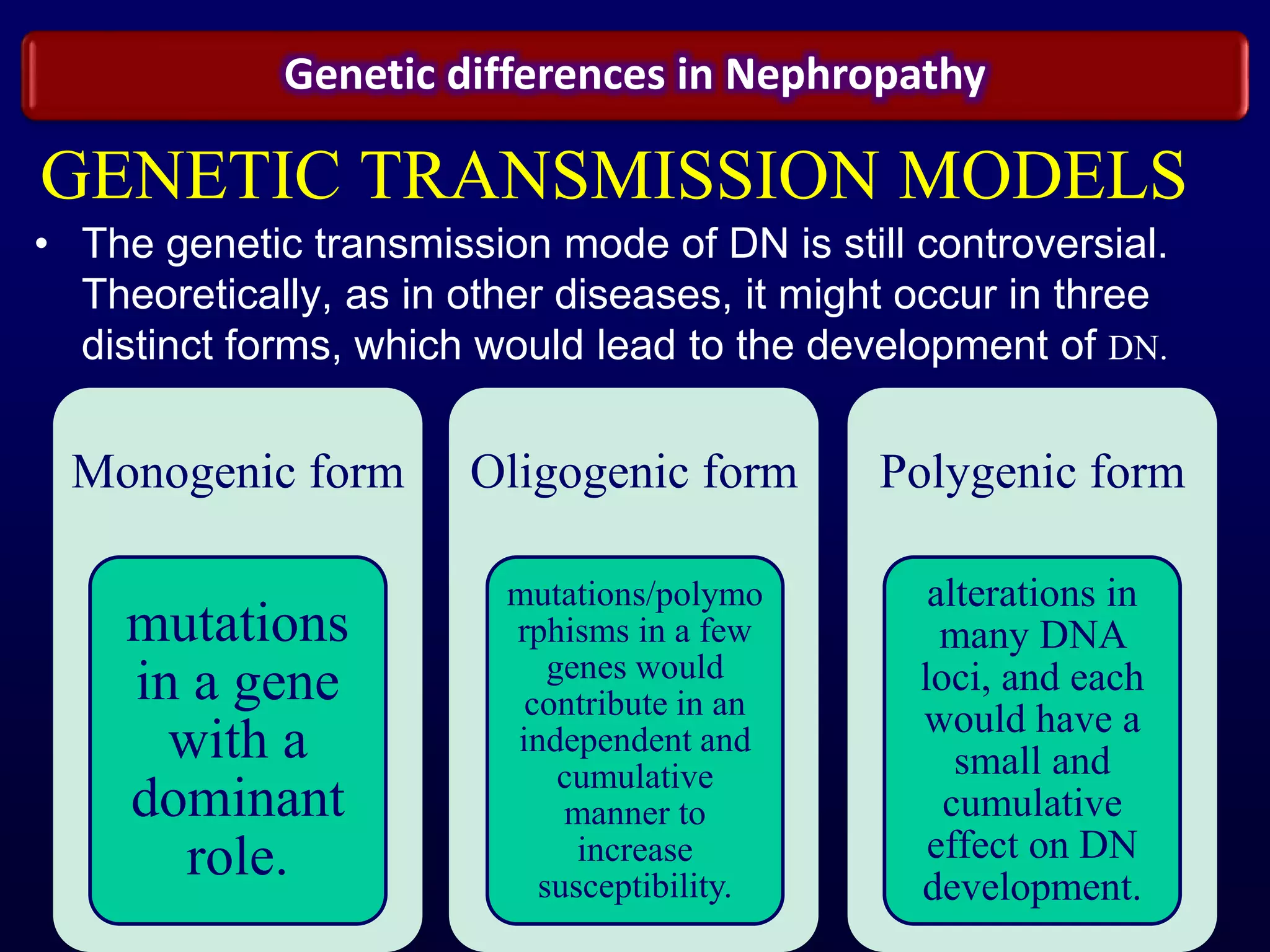
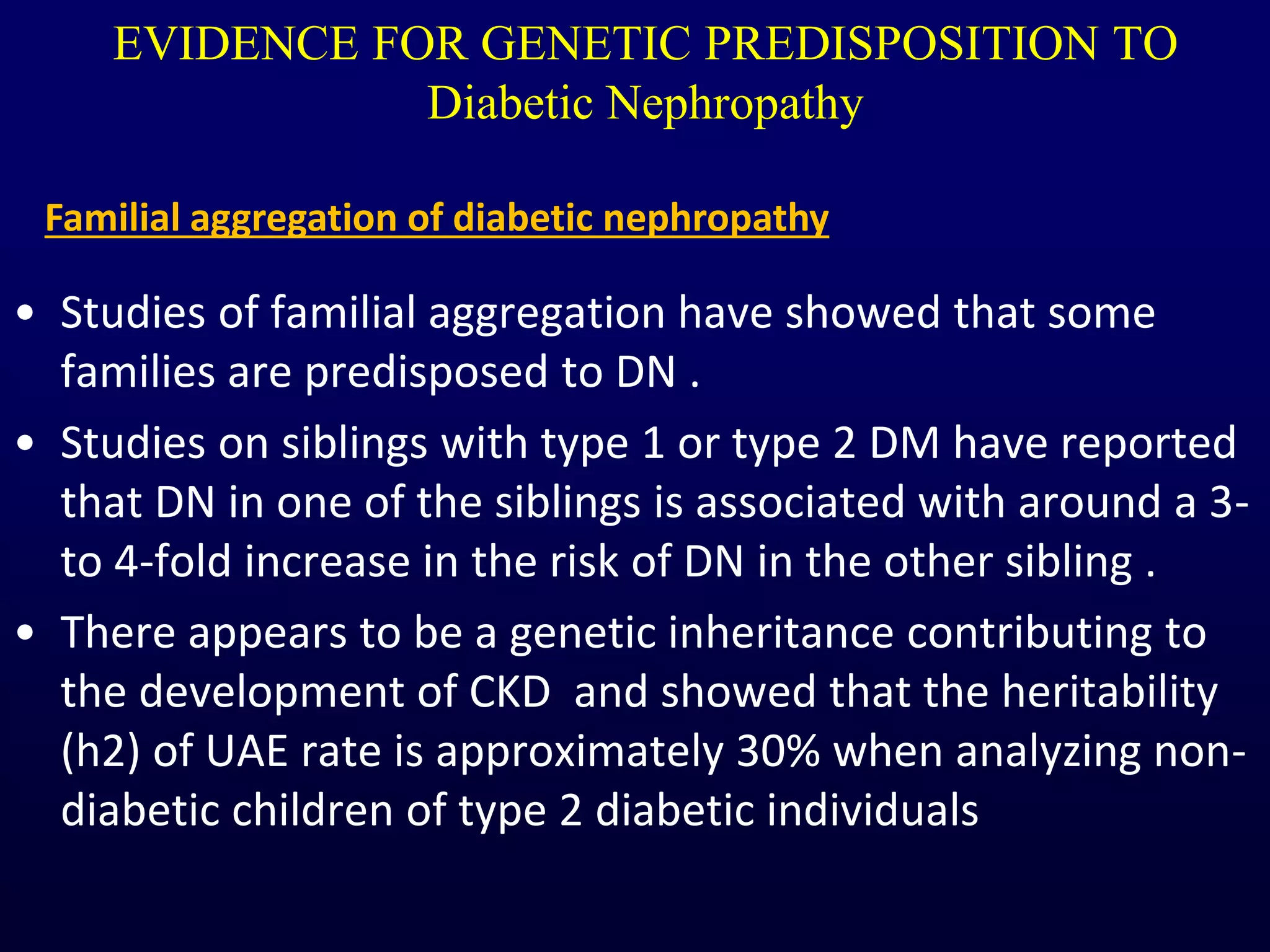
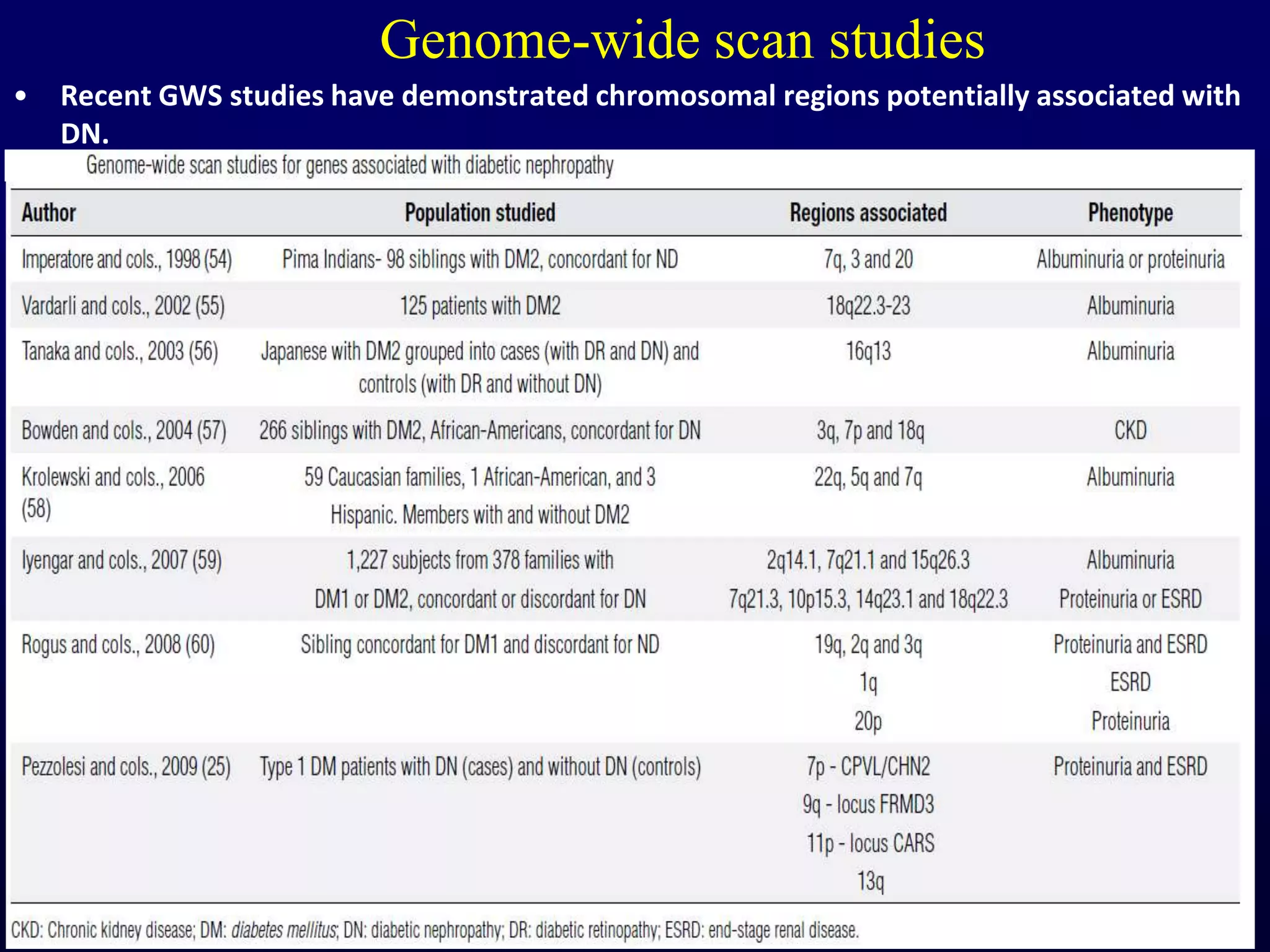
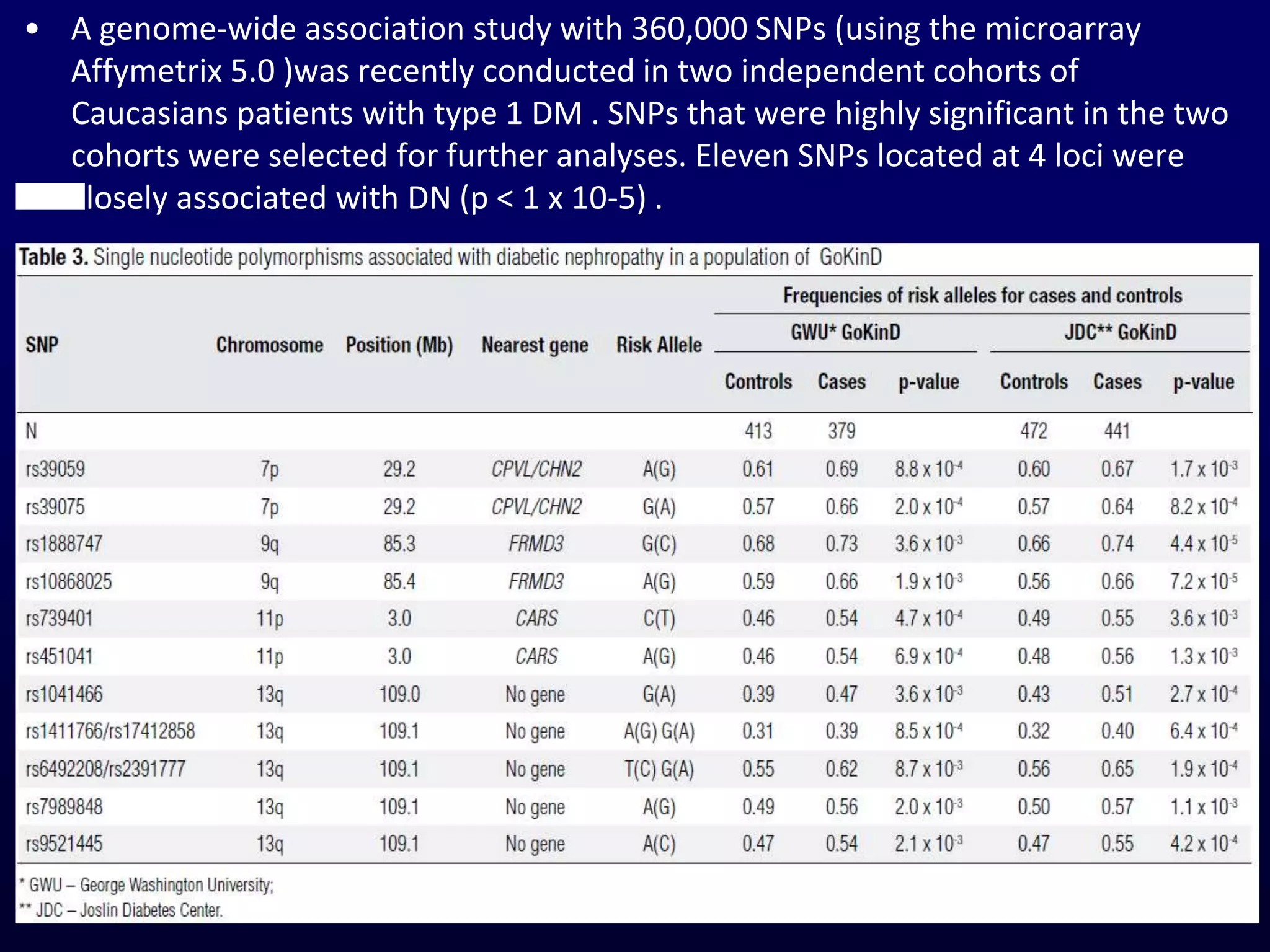
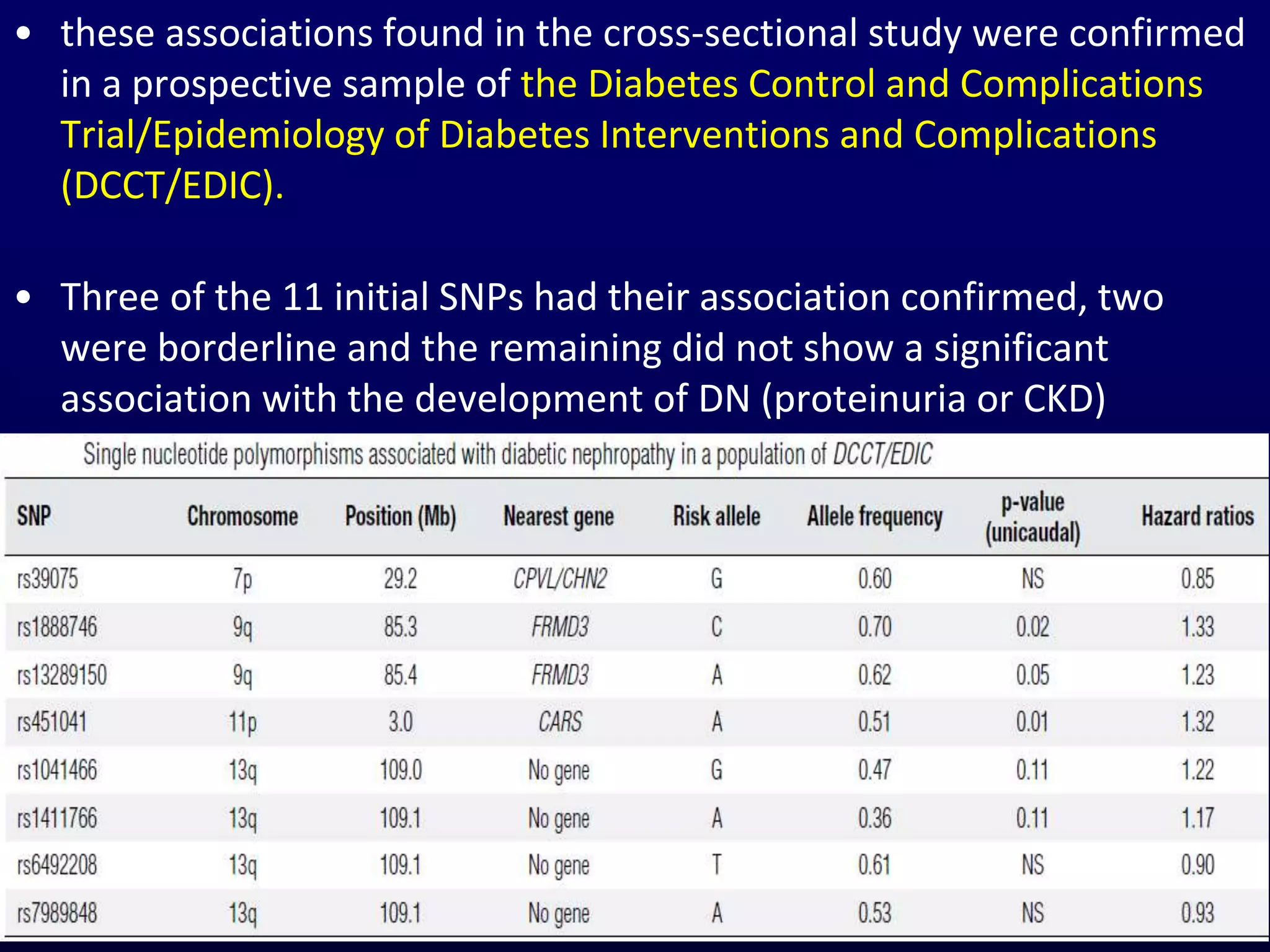
The document discusses diabetic nephropathy, a microvascular complication of diabetes characterized by albuminuria and renal function decline, leading to end-stage renal disease (ESRD), particularly prevalent in type 2 diabetes patients. It explores the epidemiology, burden, pathogenesis, genetic predispositions, and risk factors affecting the incidence and progression of diabetic nephropathy, emphasizing the importance of early identification and treatment to reduce associated health burdens. Additionally, it highlights significant gender differences in the disease's progression and the potential for genetic factors to inform preventative strategies and therapeutic choices.