Hydrogen from aluminium for vehicle propulsion
•Download as PPT, PDF•
0 likes•400 views
Hydrogen from aluminium - coming soon to a parallel universe.
Report
Share
Report
Share
Recommended
More Related Content
What's hot
What's hot (9)
Underground Coal Gasification - India & Global

Underground Coal Gasification - India & Global
Similar to Hydrogen from aluminium for vehicle propulsion
Similar to Hydrogen from aluminium for vehicle propulsion (20)
E Keane Journal Paper on Hydrogen from Aluminium Gallium

E Keane Journal Paper on Hydrogen from Aluminium Gallium
Question Answer on Energy Conservation Vol 1 By Prem Baboo.pdf

Question Answer on Energy Conservation Vol 1 By Prem Baboo.pdf
Aluminum Air Battery: How Do They Work? (Plus DIY)

Aluminum Air Battery: How Do They Work? (Plus DIY)
Production of aluminum (emphasis on energy and materials requirements) 

Production of aluminum (emphasis on energy and materials requirements)
More from Eamon Keane
More from Eamon Keane (20)
Think Global AS, "Well-to-wheel analysis of CO2 emissions in the car usage ph...

Think Global AS, "Well-to-wheel analysis of CO2 emissions in the car usage ph...
Sandia National Laboratories, "Energy Storage for the Electricity Grid: Benef...

Sandia National Laboratories, "Energy Storage for the Electricity Grid: Benef...
P. B. Andersen, "Electric vehicles in a Distributed and Integrated market usi...

P. B. Andersen, "Electric vehicles in a Distributed and Integrated market usi...
B. Mollstedt, "E.ON Mobility," in Electric Vehicle Integration Into Modern Po...

B. Mollstedt, "E.ON Mobility," in Electric Vehicle Integration Into Modern Po...
F. J. Soares, "Smart charging strategies for efficient management of the grid...

F. J. Soares, "Smart charging strategies for efficient management of the grid...
J. A. P. Lopes, "The MERGE control concept - Microgrids and EVs - Development...

J. A. P. Lopes, "The MERGE control concept - Microgrids and EVs - Development...
E. Larsen, "Efficient integration of EVs with wind power production," in Effi...

E. Larsen, "Efficient integration of EVs with wind power production," in Effi...
W. Kempton, "The Grid-Integrated Electric Vehicle," in Electric Vehicle Integ...

W. Kempton, "The Grid-Integrated Electric Vehicle," in Electric Vehicle Integ...
G. Schauer, "EV activities in Austria, EU and worldwide, Results from Fleet T...

G. Schauer, "EV activities in Austria, EU and worldwide, Results from Fleet T...
E. F. Piene, "Grid Connected Vehicles Capabilities and Characteristics," in E...

E. F. Piene, "Grid Connected Vehicles Capabilities and Characteristics," in E...
J. A. P. Lopes, "Smart EV grid interfaces responding to frequency variations ...

J. A. P. Lopes, "Smart EV grid interfaces responding to frequency variations ...
Ostkroft, "The EDISON Project," in Electric Vehicle Integration Into Modern P...

Ostkroft, "The EDISON Project," in Electric Vehicle Integration Into Modern P...
B. McBeth, "e-Mobility @ Daimler," in Electric Vehicle Integration Into Moder...

B. McBeth, "e-Mobility @ Daimler," in Electric Vehicle Integration Into Moder...
The Effect of Wind Generation on Combined Heat and Power

The Effect of Wind Generation on Combined Heat and Power
Recently uploaded
Mehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...Nguyen Thanh Tu Collection
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
Recently uploaded (20)
Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi

Russian Escort Service in Delhi 11k Hotel Foreigner Russian Call Girls in Delhi
TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...

TỔNG ÔN TẬP THI VÀO LỚP 10 MÔN TIẾNG ANH NĂM HỌC 2023 - 2024 CÓ ĐÁP ÁN (NGỮ Â...
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...

Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...
UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf

UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Hydrogen from aluminium for vehicle propulsion
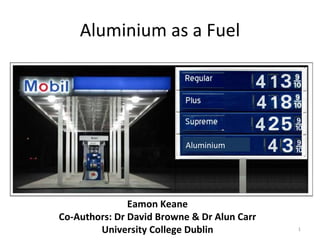
- 1. Aluminium as a Fuel Aluminium Eamon Keane Co-Authors: Dr David Browne & Dr Alun Carr University College Dublin
- 2. Water + Aluminium-Gallium Fuel Pellets
- 4. = 1L 2.2L
- 6. + = + Water Hydrogen on Demand Aluminium Hydroxide
- 7. Aluminium Fuel Cycle Inputs
- 8. Calcining Smelting Aluminium Fuel Cycle 13.5% energy efficiency, not 50% Aluminium Hydroxide Alumina
- 9. Energy and CO 2 Per Km Travelled
- 10. Pourbaix Diagram
- 11. Aluminium-Gallium Phase Diagram 33um Pure gallium in grain boundaries, β phase α phase: solid solution
- 12. Fuel Pellet The Reaction Gallium is not inert in the reaction
- 13. Standard Electrode Potential Large difference, galvanic corrosion expected. Reaction Mechanism Element E°V (V) Lithium -3.04 Aluminium -1.66 Gallium -0.56 Hydrogen 0 Gold +0.80
- 17. Gallium Production Enough for ~ 30,000 cars – not 1 billion!
- 18. Elements with very low worldwide 2008 production (Up to ~ 10,000 tons)
- 19. Aluminium as a Fuel Aluminium Any Questions? Renewable ≠ Green