Report
Share
Recommended
Recommended
More Related Content
What's hot
What's hot (20)
Microspheres - Methods for Preparation of Microspheres

Microspheres - Methods for Preparation of Microspheres
BIOPHARMACEUTICS OF ANTISENSE MOLECULE AND APTAMERS

BIOPHARMACEUTICS OF ANTISENSE MOLECULE AND APTAMERS
Viewers also liked
Viewers also liked (20)
Protein and peptide drug delivery seminar-97-2003-final2

Protein and peptide drug delivery seminar-97-2003-final2
Similar to liposomes
Targeted Drug Delivery System Unit-IV in B Pharm IV syllabus Targeted Drug Delivery System Unit-IV DrNitalikar

Targeted Drug Delivery System Unit-IV DrNitalikarRajarambapu College of Pharmacy Kasegaon Dist Sangli
Similar to liposomes (20)
More from Danish Kurien
More from Danish Kurien (13)
Parenteral controlled drug delivery system sushmitha

Parenteral controlled drug delivery system sushmitha
Recently uploaded
https://app.box.com/s/x7vf0j7xaxl2hlczxm3ny497y4yto33i80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...

80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...Nguyen Thanh Tu Collection
Making communications land - Are they received and understood as intended? webinar
Thursday 2 May 2024
A joint webinar created by the APM Enabling Change and APM People Interest Networks, this is the third of our three part series on Making Communications Land.
presented by
Ian Cribbes, Director, IMC&T Ltd
@cribbesheet
The link to the write up page and resources of this webinar:
https://www.apm.org.uk/news/making-communications-land-are-they-received-and-understood-as-intended-webinar/
Content description:
How do we ensure that what we have communicated was received and understood as we intended and how do we course correct if it has not.Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...Association for Project Management
Recently uploaded (20)
Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...

Kodo Millet PPT made by Ghanshyam bairwa college of Agriculture kumher bhara...
HMCS Vancouver Pre-Deployment Brief - May 2024 (Web Version).pptx

HMCS Vancouver Pre-Deployment Brief - May 2024 (Web Version).pptx
80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...

80 ĐỀ THI THỬ TUYỂN SINH TIẾNG ANH VÀO 10 SỞ GD – ĐT THÀNH PHỐ HỒ CHÍ MINH NĂ...
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Making communications land - Are they received and understood as intended? we...

Making communications land - Are they received and understood as intended? we...
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
On National Teacher Day, meet the 2024-25 Kenan Fellows

On National Teacher Day, meet the 2024-25 Kenan Fellows
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...

Sensory_Experience_and_Emotional_Resonance_in_Gabriel_Okaras_The_Piano_and_Th...
Interdisciplinary_Insights_Data_Collection_Methods.pptx

Interdisciplinary_Insights_Data_Collection_Methods.pptx
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
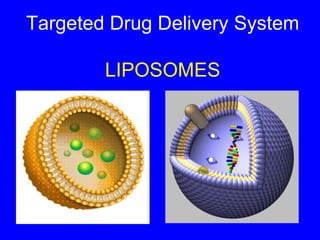
liposomes
- 1. Targeted Drug Delivery System LIPOSOMES
- 17. Methods of liposomes preparations Passive loading technique Active loading technique Mechanical dispersion methods Solvent dispersion methods Detergent removal methods
- 20. Physical Characterization Surface charge Free-flow electrophoresis Electrical surface potential and surface pH Zetapotential measurements & pH sensitive probes Percent of free drug/ percent capture Drug release Diffusion cell/ dialysis Parameter Characterization method Vesicle shape and surface morphology Mean vesicle size and size distribution Dynamic light scattering, zetasizer, Photon correlation spectroscopy, laser light scattering, gel permeation and gel exclusion Minicolumn centrifugation, ion-exchange chromatography, radiolabelling Transmission electron microscopy, Freeze-fracture electron microscopy
- 21. Phopholipid peroxidation UV absorbance, Iodometric and GLC Phospholipid hydrolysis, Cholesterol auto-oxidation HPLC and TLC Osmolarity Parameter Characterization method Phospholipid concentration Cholesterol concentration Cholesterol oxidase assay and HPLC Osmometer Barlett assay, stewart assay, HPLC Chemical Characterization
- 22. Biological Characterization Animal toxicity Monitoring survival rates, histology and pathology Parameter Characterization method Sterility Pyrogenicity Limulus Amebocyte Lysate (LAL) test Aerobic or anaerobic cultures
- 28. Pseudomonas infection, aeruginosa Pulmonary delivery Tobramycin Asthma Pulmonary delivery Pentoxyfylline Pain muscle condition Ocular delivery Ketoprofen Diabetic mellitus Oral, Ocular, Pulmonary and Transdermal delivery Insulin Mycotic infection Oral delivery Amphotericin-B Targeted Diseases Route of administration Drug
- 29. Cancer Transdermal Methotrexate Meningococal, staphylococcal Pulmonary delivery Penicillin G Glucoma, Conjectivitis Ocular delivery Adrenaline Rheumatoid arthritis Oral delivery Ibuprofen ulcer on mucous surface with pain Transdermal Benzocain Asthma Pulmonary delivery Salbutamol Targeted Diseases Route of administration Drug
- 30. Britannia Pharm, UK Expanding lung diseases in babies Dry protein free powder of DPPC-PG ALECTM The liposome company, USA Systemic inflammatory diseases Prostaglandin-E1 VENTUSTM SEQUUS, USA fungal infections, Leishmaniasis Amphotericin-B AmphotecTM NeXstar, USA Kaposi’s sarcoma, breast & lung cancer Daunorubicin DaunoXomeTM SEQUUS, USA Kaposi’s sarcoma Doxorubicin DoxilTM or CaelyxTM Company Target diseases Drug used Marketed product
- 31. Swiss serum & vaccine institute, Switzerland Hepatitis A Inactivated hepatitis-A Virions Epaxal –Berna Vaccine Vineland lab, USA Chicken pox Killed avian retrovirus Avian retrovirus vaccine Novavax, USA Smallpox Smallpox vaccine Novasome® Skye Pharm, USA Cancer therapy Cytarabine Depocyt Ozone, USA Asthma Terbutaline sulphate Topex-Br Company Target diseases Drug used Marketed product
- 35. Any question...
- 36. Thank You
