Report
Share
Recommended
Recommended
More Related Content
Similar to Notechapter2
Similar to Notechapter2 (20)
More from Zahari Rusdi
More from Zahari Rusdi (20)
Recently uploaded
💉💊+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHABI}}+971581248768
+971581248768 Mtp-Kit (500MG) Prices » Dubai [(+971581248768**)] Abortion Pills For Sale In Dubai, UAE, Mifepristone and Misoprostol Tablets Available In Dubai, UAE CONTACT DR.Maya Whatsapp +971581248768 We Have Abortion Pills / Cytotec Tablets /Mifegest Kit Available in Dubai, Sharjah, Abudhabi, Ajman, Alain, Fujairah, Ras Al Khaimah, Umm Al Quwain, UAE, Buy cytotec in Dubai +971581248768''''Abortion Pills near me DUBAI | ABU DHABI|UAE. Price of Misoprostol, Cytotec” +971581248768' Dr.DEEM ''BUY ABORTION PILLS MIFEGEST KIT, MISOPROTONE, CYTOTEC PILLS IN DUBAI, ABU DHABI,UAE'' Contact me now via What's App…… abortion Pills Cytotec also available Oman Qatar Doha Saudi Arabia Bahrain Above all, Cytotec Abortion Pills are Available In Dubai / UAE, you will be very happy to do abortion in Dubai we are providing cytotec 200mg abortion pill in Dubai, UAE. Medication abortion offers an alternative to Surgical Abortion for women in the early weeks of pregnancy. We only offer abortion pills from 1 week-6 Months. We then advise you to use surgery if its beyond 6 months. Our Abu Dhabi, Ajman, Al Ain, Dubai, Fujairah, Ras Al Khaimah (RAK), Sharjah, Umm Al Quwain (UAQ) United Arab Emirates Abortion Clinic provides the safest and most advanced techniques for providing non-surgical, medical and surgical abortion methods for early through late second trimester, including the Abortion By Pill Procedure (RU 486, Mifeprex, Mifepristone, early options French Abortion Pill), Tamoxifen, Methotrexate and Cytotec (Misoprostol). The Abu Dhabi, United Arab Emirates Abortion Clinic performs Same Day Abortion Procedure using medications that are taken on the first day of the office visit and will cause the abortion to occur generally within 4 to 6 hours (as early as 30 minutes) for patients who are 3 to 12 weeks pregnant. When Mifepristone and Misoprostol are used, 50% of patients complete in 4 to 6 hours; 75% to 80% in 12 hours; and 90% in 24 hours. We use a regimen that allows for completion without the need for surgery 99% of the time. All advanced second trimester and late term pregnancies at our Tampa clinic (17 to 24 weeks or greater) can be completed within 24 hours or less 99% of the time without the need surgery. The procedure is completed with minimal to no complications. Our Women's Health Center located in Abu Dhabi, United Arab Emirates, uses the latest medications for medical abortions (RU-486, Mifeprex, Mifegyne, Mifepristone, early options French abortion pill), Methotrexate and Cytotec (Misoprostol). The safety standards of our Abu Dhabi, United Arab Emirates Abortion Doctors remain unparalleled. They consistently maintain the lowest complication rates throughout the nation. Our Physicians and staff are always available to answer questions and care for women in one of the most difficult times in their lives. The decision to have an abortion at the Abortion Cl+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...

+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...?#DUbAI#??##{{(☎️+971_581248768%)**%*]'#abortion pills for sale in dubai@
Recently uploaded (20)
CNIC Information System with Pakdata Cf In Pakistan

CNIC Information System with Pakdata Cf In Pakistan
TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery

TrustArc Webinar - Unlock the Power of AI-Driven Data Discovery
Finding Java's Hidden Performance Traps @ DevoxxUK 2024

Finding Java's Hidden Performance Traps @ DevoxxUK 2024
Elevate Developer Efficiency & build GenAI Application with Amazon Q

Elevate Developer Efficiency & build GenAI Application with Amazon Q
Mcleodganj Call Girls 🥰 8617370543 Service Offer VIP Hot Model

Mcleodganj Call Girls 🥰 8617370543 Service Offer VIP Hot Model
Web Form Automation for Bonterra Impact Management (fka Social Solutions Apri...

Web Form Automation for Bonterra Impact Management (fka Social Solutions Apri...
How to Troubleshoot Apps for the Modern Connected Worker

How to Troubleshoot Apps for the Modern Connected Worker
Repurposing LNG terminals for Hydrogen Ammonia: Feasibility and Cost Saving

Repurposing LNG terminals for Hydrogen Ammonia: Feasibility and Cost Saving
Modular Monolith - a Practical Alternative to Microservices @ Devoxx UK 2024

Modular Monolith - a Practical Alternative to Microservices @ Devoxx UK 2024
Apidays New York 2024 - APIs in 2030: The Risk of Technological Sleepwalk by ...

Apidays New York 2024 - APIs in 2030: The Risk of Technological Sleepwalk by ...
+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...

+971581248768>> SAFE AND ORIGINAL ABORTION PILLS FOR SALE IN DUBAI AND ABUDHA...
ICT role in 21st century education and its challenges

ICT role in 21st century education and its challenges
Connector Corner: Accelerate revenue generation using UiPath API-centric busi...

Connector Corner: Accelerate revenue generation using UiPath API-centric busi...
Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe

Apidays New York 2024 - Scaling API-first by Ian Reasor and Radu Cotescu, Adobe
Apidays New York 2024 - Passkeys: Developing APIs to enable passwordless auth...

Apidays New York 2024 - Passkeys: Developing APIs to enable passwordless auth...
Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood

Polkadot JAM Slides - Token2049 - By Dr. Gavin Wood
Notechapter2
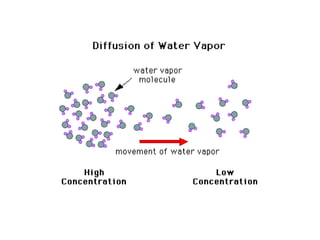
- 2. Diffusion of bromine gas Experiment diffusion of bromine in air The diffusion of bromine progresses Air colourless ______________________ spreads through the first cylinder
- 3. ___________________________ The reddish-brown vapor moves upward, slowly filling the ___________________ Reddish-brown vapour spreads through the __________________
- 4. Kinetic Theory of Matter The _________________ and _________________ of ______________________ in
- 8. ________ More than in _______ but less than in _______ _________ _________ contents of the particles Low _________ _________ _________ GAS LIQUIDS SOLIDS Properties
- 10. CHANGES IN THE STATE OF MATTER ___________________ ____________________ EVAPORATION or BOILING _____________________ _____________________
- 11. INTER-CONVERSION OF THE STATES OF MATTER
- 12. MELTING When a _________ is heated The particles in the solid __________________ _____________and _______________________ The particles vibrate faster as the __________ _________until the energy they gained is able to ____________________ that hold them at their _______________________ At this point the __________ becomes _________________ Process _______________ HEAT SOLID LIQUID The temperature at this point: _______________
- 14. FREEZING When a __________ is _____________ The particles in the liquid ______________ __________and ______________________ As the _____________ continues to ________, the particles continue to _________________ until they do not enough energy to ___________________ At this point the _____________ changes into a ________________ Process ________________ The temperature at this point: ______________________________
- 16. CHANGE IN THE STATE OF MATTER IN NAPHTHALENE HEATING CURVE OF NAPHTALENE Melting point -------- A Solid B C Start to melt Melt completely D
- 17. At point ____, naphthalene exists as ___________ When _______ is heated, ______________ is absorbed that cause particles to _______________ and _____________ ( This is why temperature ______________ from point _____ to point _____) At point ___, solid naphthalene begins to __________ During the ____________, the temperature of naphthalene does not rise even though heating continues because heat energy ____________ by the particles is used to _____________ the ____________________ so that _______________ can turn into ___________
- 18. This constant temperature: ____________________ of Naphthalene Both ______________ and ___________ are present At point _____, ________________ naphthalene has ____________ From point _____ to point ______, the particles in liquid naphthalene absorb heat energy and move faster ____________________
