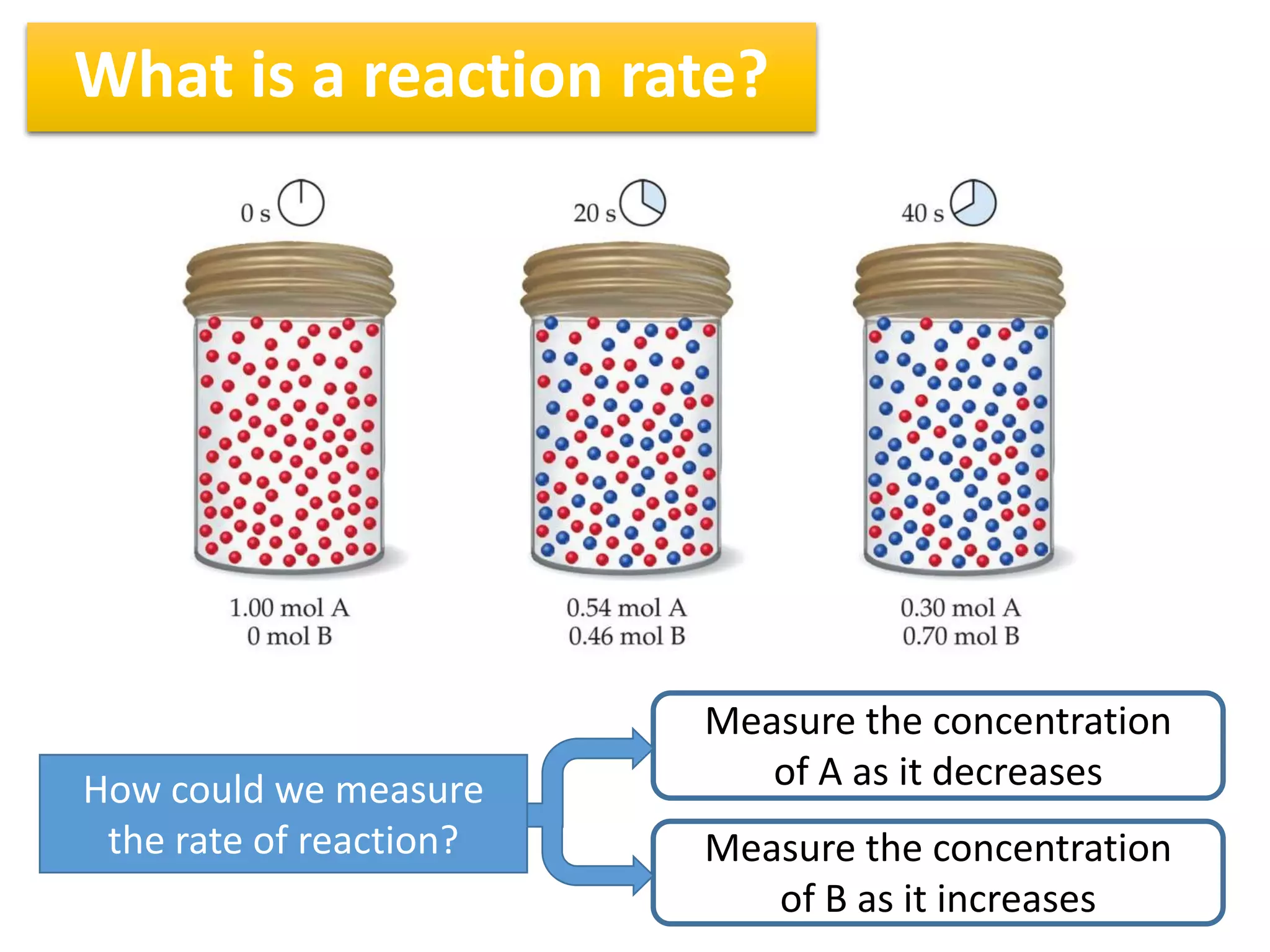
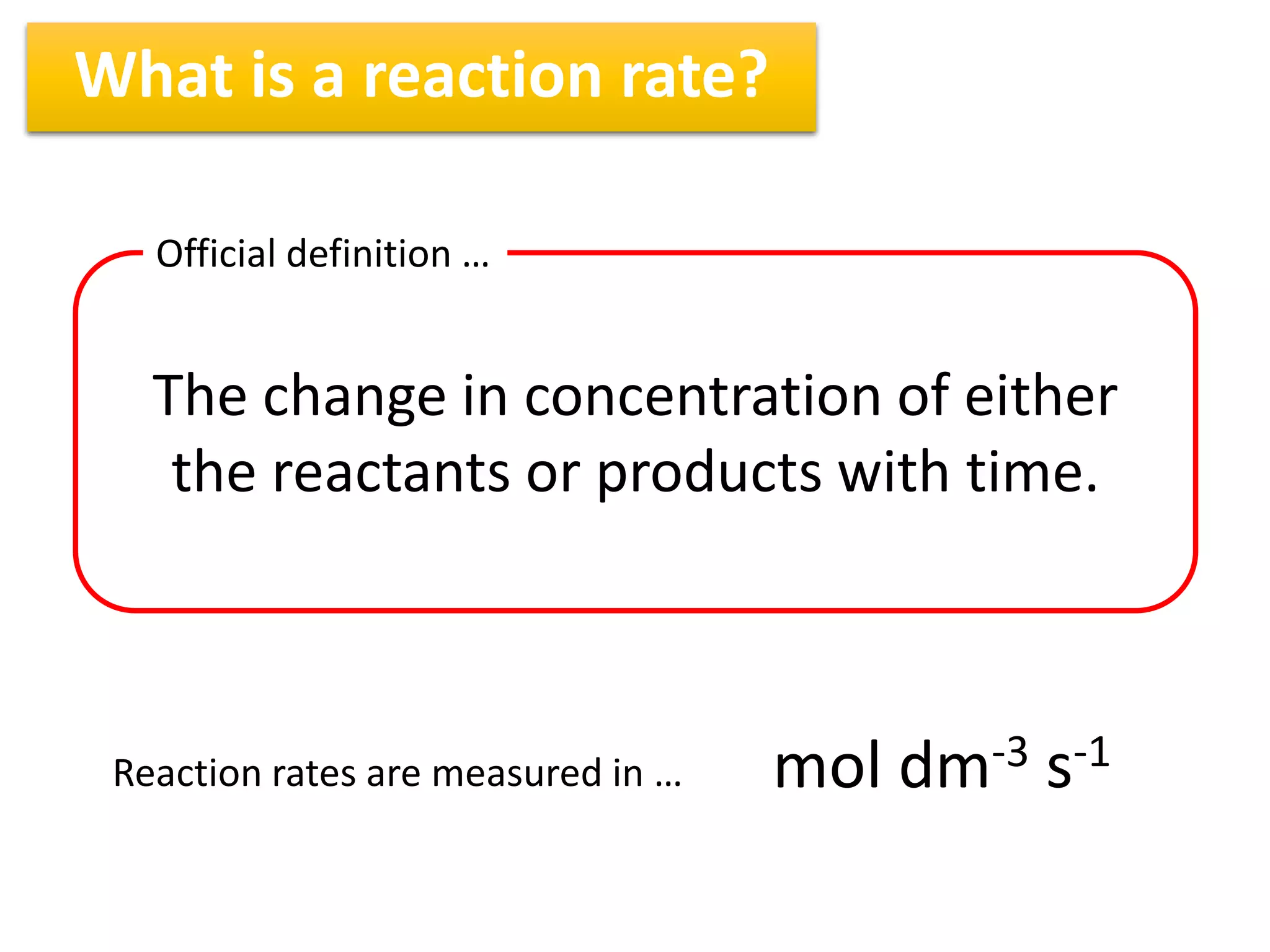
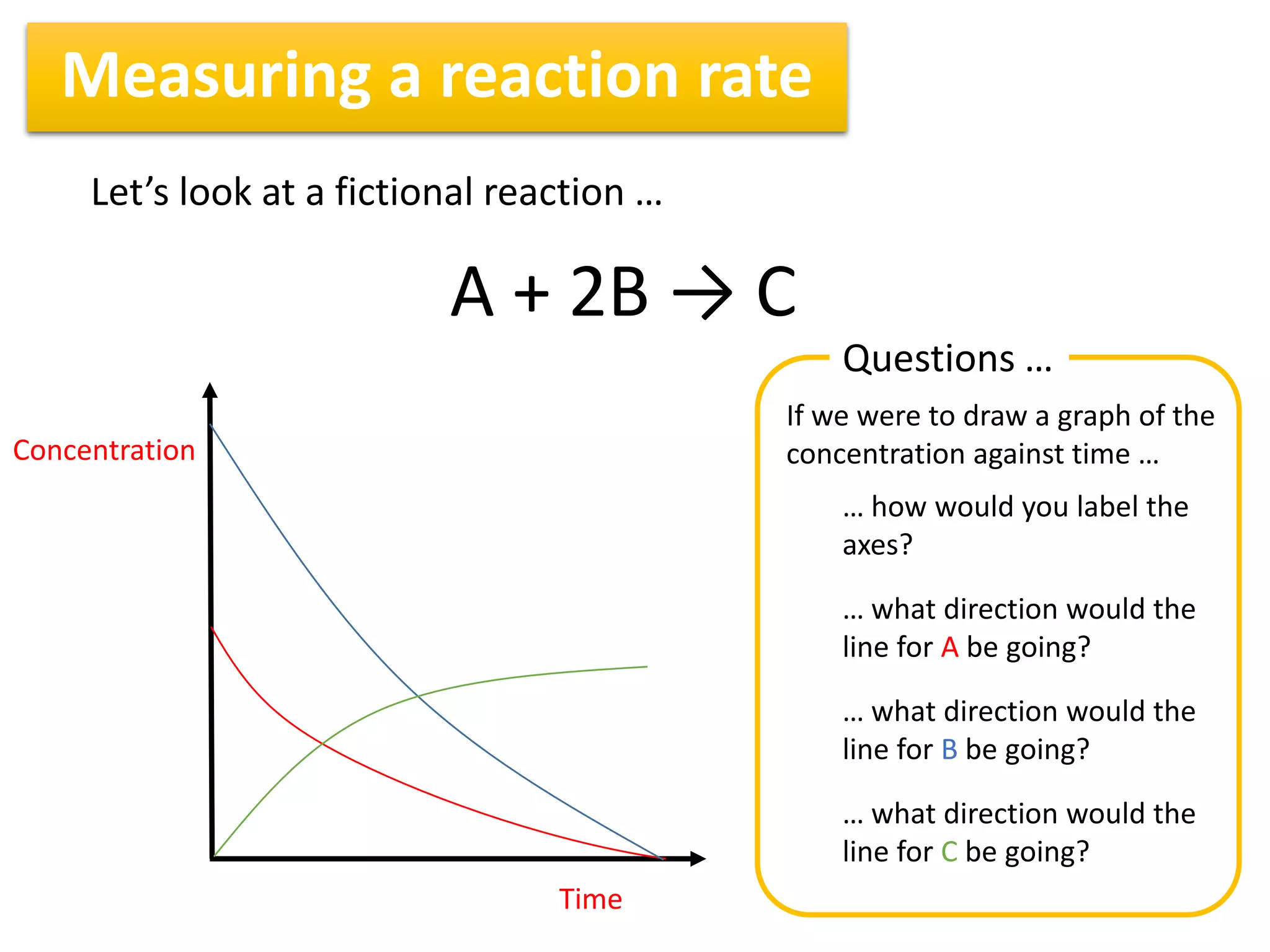
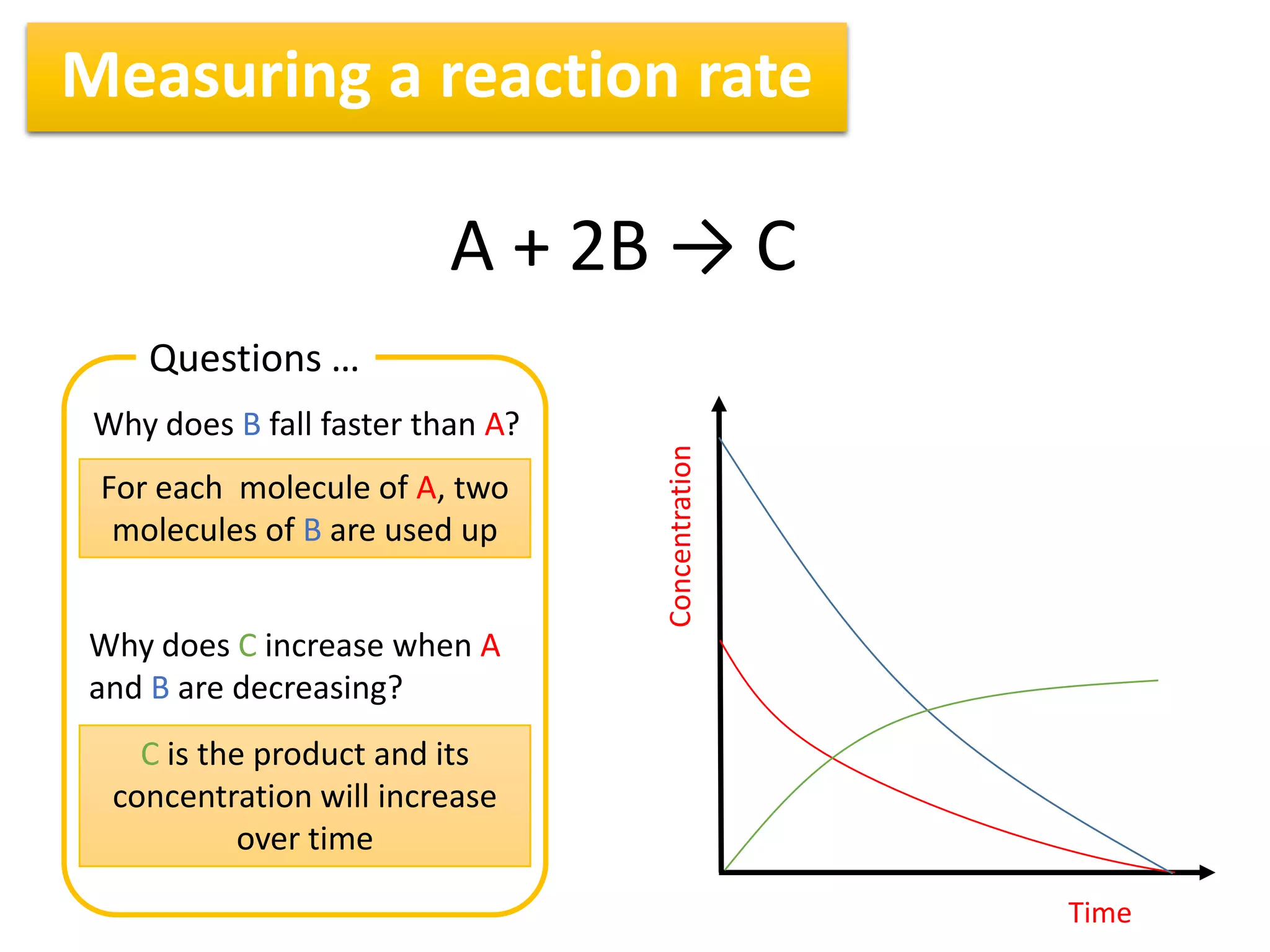
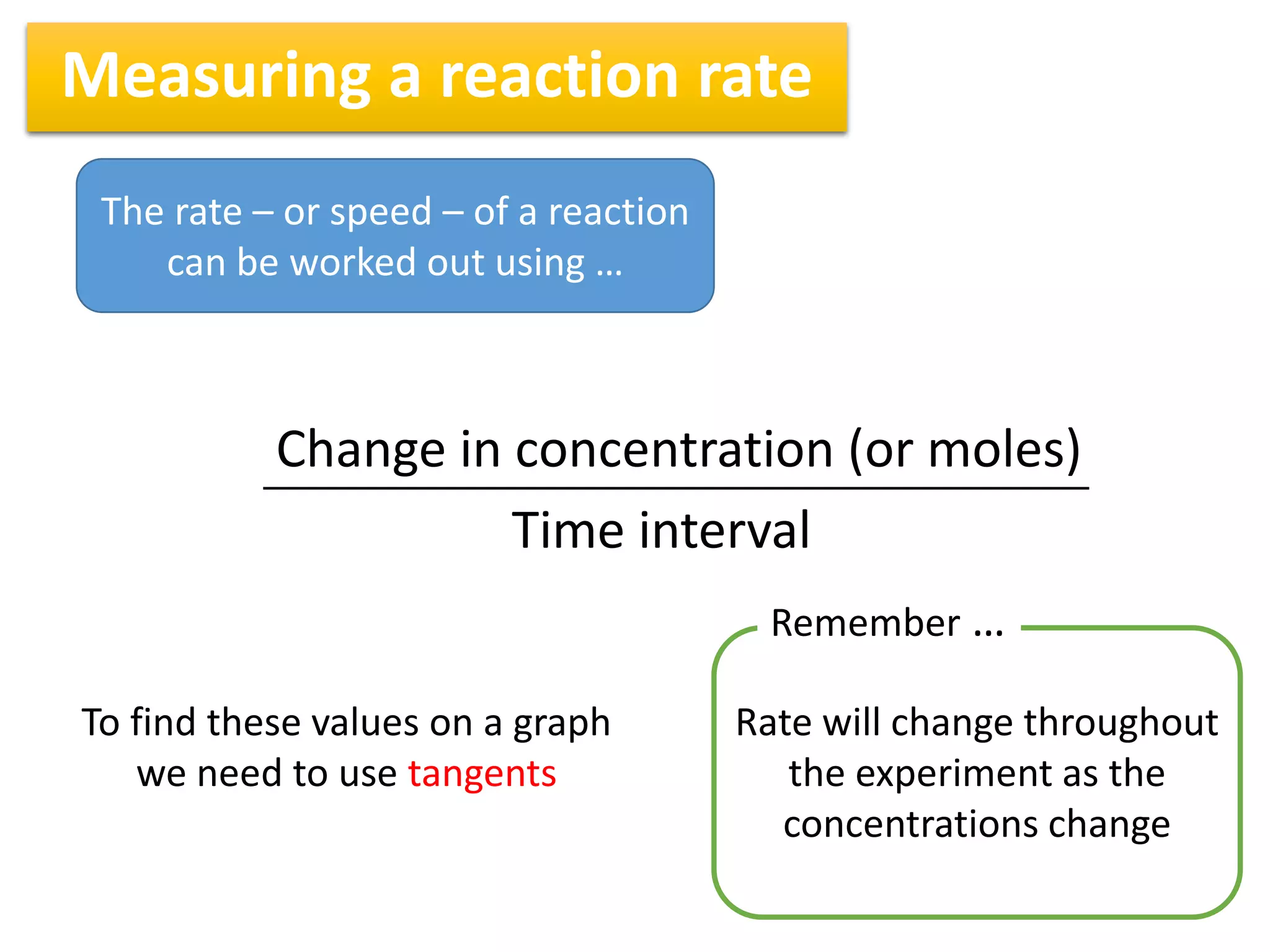
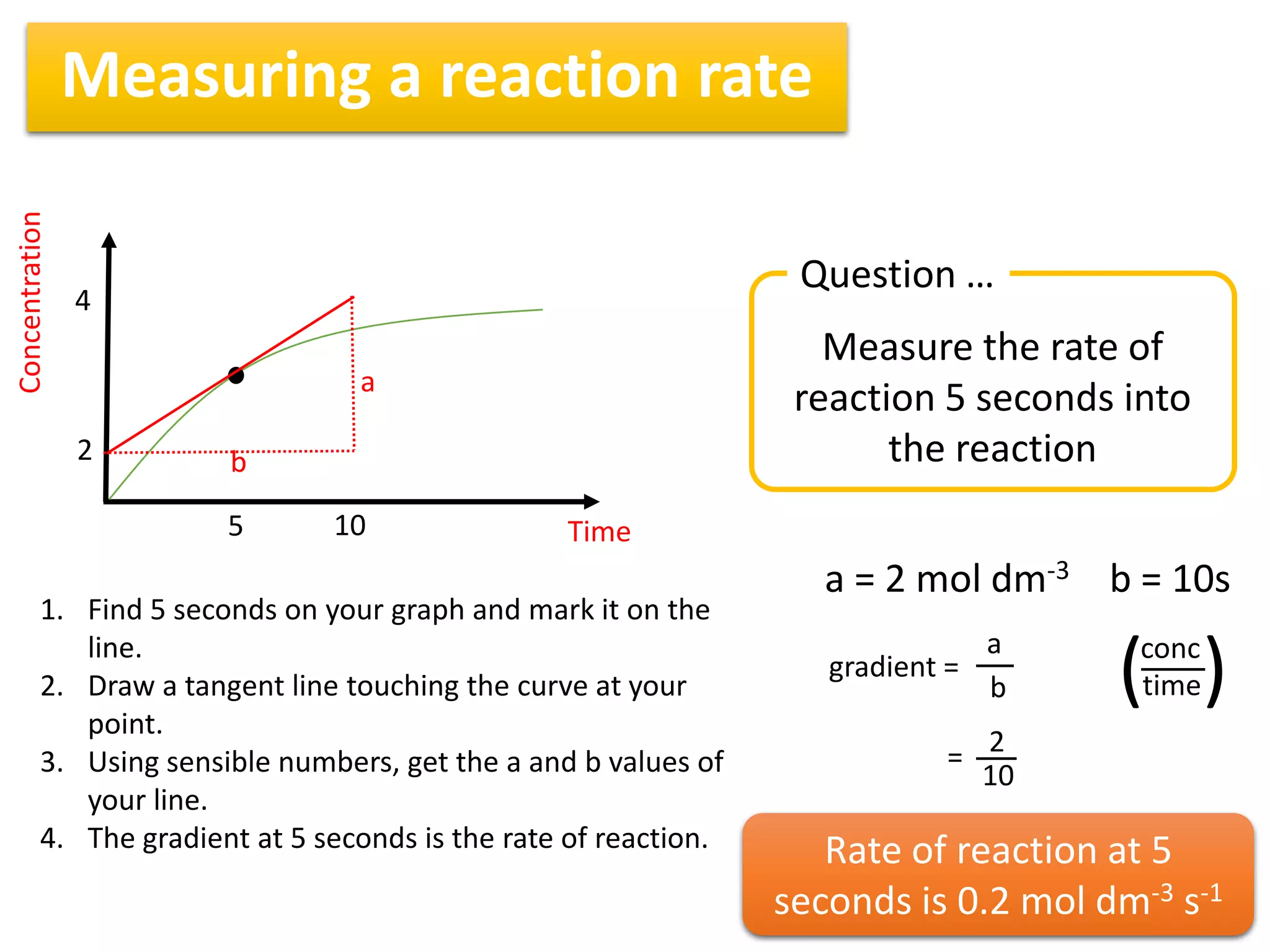
1) Reaction rates can be measured by monitoring the change in concentration of reactants or products over time. For the reaction A + 2B → C, the concentration of A would decrease over time while concentrations of B and C would increase.
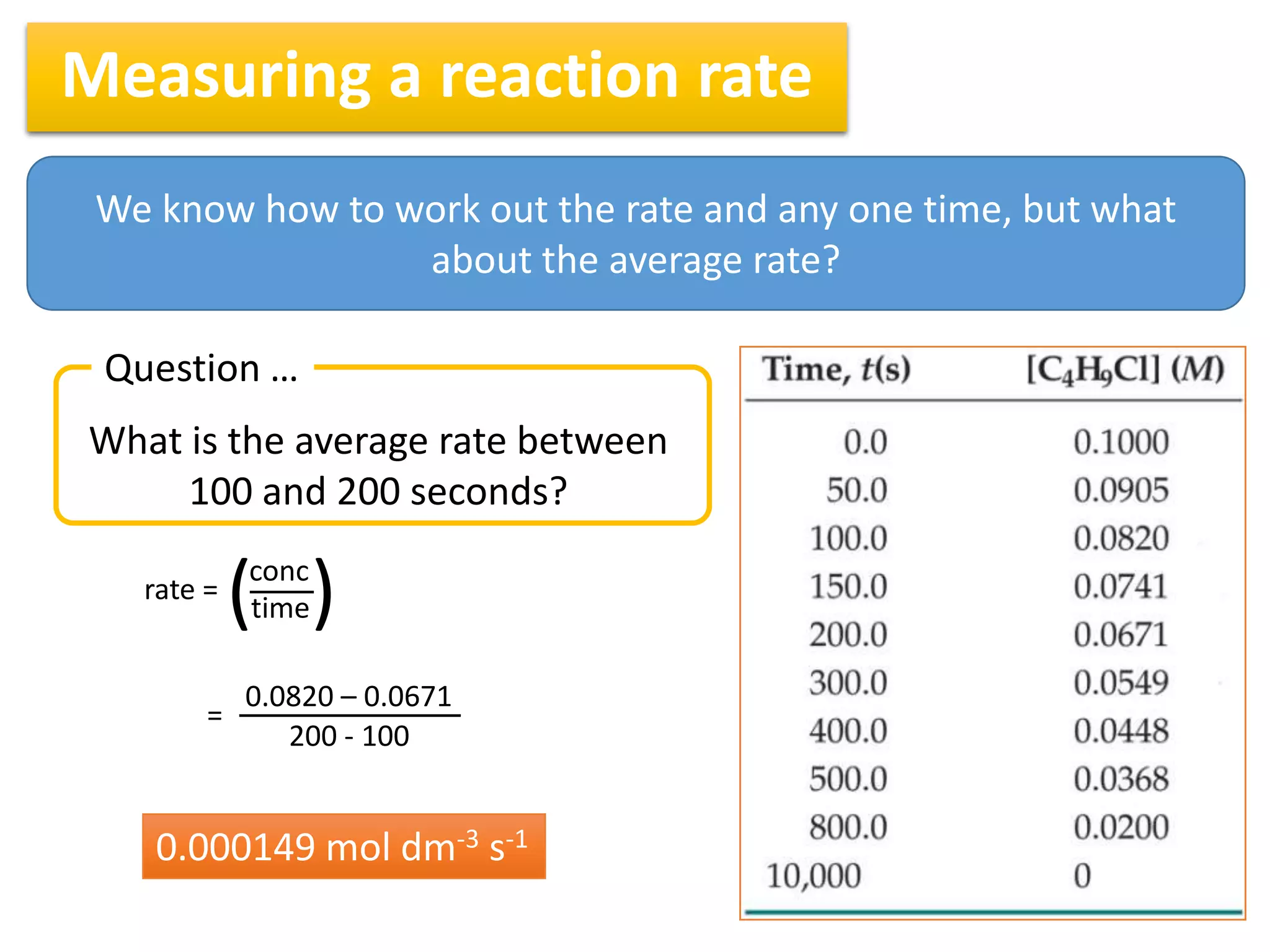
2) The rate of a reaction is calculated by taking the change in concentration divided by the change in time between two measurements. For example, the average rate between 100 and 200 seconds can be found by taking the change in concentration over that time interval.
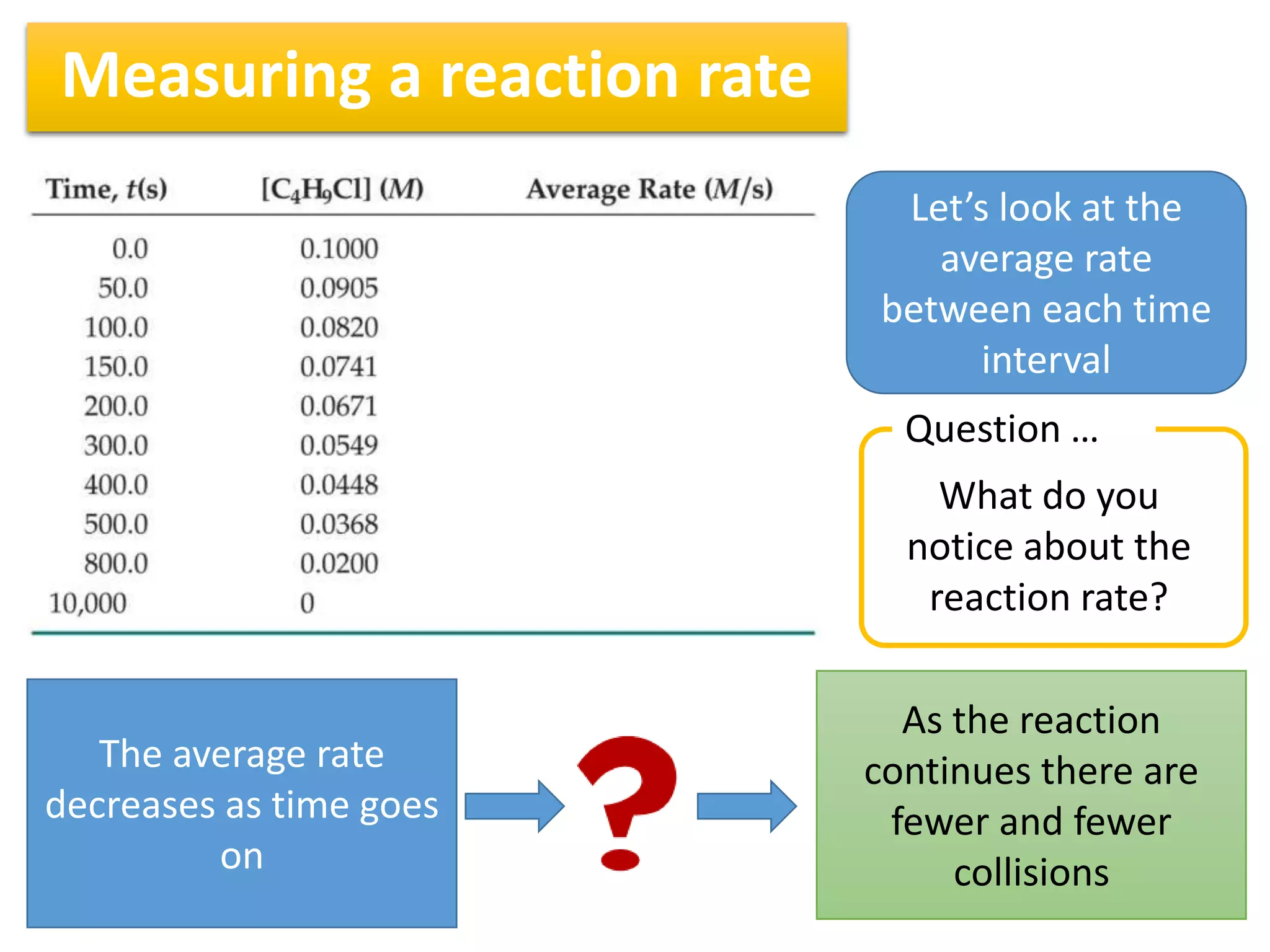
3) Reaction rates typically decrease over time as there are fewer collisions between reactants as their concentrations decrease. Measuring rates at different time intervals shows the average rate decreasing as the reaction progresses.