IB Chemistry on Stereoisomers, E/Z, Cis Trans, Geometric, Optical and Polarimetry
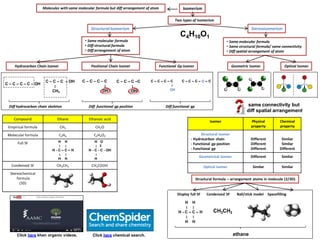
- 1. IsomerismMolecules with same molecular formula but diff arrangement of atom Two types of Isomerism Positional Chain Isomer Functional Gp Isomer C – C – C – C – OH C4H10O1 StructuralIsomerism • Same molecular formula • Diff structural formula • Diff arrangement of atom Diff hydrocarbon chain skeleton • Same molecular formula • Same structural formula/ same connectivity • Diff spatial arrangement of atom Stereoisomerism Hydrocarbon Chain Isomer Diff functional gp position Diff functional gp C – C – C – OH ׀ CH3 C – C – C –C ׀ OH C – C – C – C ׀ OH C – C – C – C ׀ OH C – C – C – O – C Optical IsomerGeometric Isomer Click here khan organic videos. Compound Ethane Ethanoic acid Empirical formula CH3 CH2O Molecular formula C2H6 C2H4O2 Full SF Condensed SF CH3CH3 CH3COOH Stereochemical formula (3D) Isomer Physical property Chemical property Structural isomer - Hydrocarbon chain - Functional gp position - Functional gp Different Different Different Similar Similar Different Geometrical isomer Different Similar Optical isomer Similar Similar H H ׀ ׀ H - C – C – H ׀ ׀ H H H O ׀ ‖ H - C - C - OH ׀ H Structural formula – arrangement atoms in molecule (2/3D) H H ׀ ׀ H - C – C – H ׀ ׀ H H CH3CH3 ethane Display full SF Condensed SF Ball/stick model Spacefilling Click here chemical search. same connectivity but diff spatial arrangement
- 2. CI Isomerism Two types of Isomerism Positional Chain Isomer Functional Gp Isomer C4H10O1 StructuralIsomerism • Same molecular formula • Diff structural formula • Diff arrangement of atom • Same molecular formula • Same structural formula/same connectivity • Diff spatial arrangement of atom Stereoisomerism Hydrocarbon Chain Isomer Optical IsomerGeometric Isomer Requirement for geometric isomers • Presence of C=C double bond - prevent bond rotation • Presence of ring structure- prevent bond rotation • Carbon atom in double bond bonded to diff atom Cis Isomers Atom located on same side Trans Isomers Atom located on diff side Presence C=C Presence of ring structure CI CI ׀ ׀ C = C ׀ ׀ H H Cis 1, 2 dichloroethene CI H ׀ ׀ C = C ׀ ׀ H CI Trans 1, 2 dichloroethene Cis 1, 2 dichlorocyclopropane Trans 1, 2 dichlorocyclopropane Carbon atom bond to diff atom double bond C – C – C – C – OH C – C – C –C ׀ OH C – C – C – O – C C – C – C – OH ׀ CH3 CI CI CI CI H HHH CI CI H H H H CI Cis 1, 3 dichlorocyclobutane Trans 1, 3 dichlorocyclobutane same atom different atomχ ✓ No geometric isomers Geometric isomers same connectivity but diff spatial arrangement
- 3. • Same molecular formula • Same structural formula/same connectivity • Diff spatial arrangement of atom Stereoisomerism Geometric Isomer Requirement for geometric isomers • Presence of C=C double bond - prevent bond rotation • Presence of ring structure - prevent bond rotation • Carbon atom in double bond bond to diff atom Cis Isomers Atom located on same side Trans Isomers Atom located on diff side Presence C=C Presence of ring structure CI CI ׀ ׀ C = C ׀ ׀ H H Cis 1, 2 dichloroethene CI H ׀ ׀ C = C ׀ ׀ H CI Trans 1, 2 dichloroethene Cis 1, 2 dichlorocyclopropane Trans 1, 2 dichlorocyclopropane Carbon atom bond to diff atom double bond CI CI CI CI H HHH same atom different atom Cis / Trans naming sys click here E/Z naming system 2 diff substituent on each carbon [Z] 1, 2 dichloroethene [E] 1, 2 dichloroethene CI CI ׀ ׀ C = C ׀ ׀ H H CI H ׀ ׀ C = C ׀ ׀ H CI Atom same side Atom opposite side [Z] 1, 2 dichlorocyclopropane [E] 1, 2 dichlorocyclopropane E / Z naming sys E/Z (CIP priority) 4 diff substituents 1 1 – high priority 2 – low priority 1 2 1 2 2 2 1 Priority same side – Z Priority opp side - E H H CICI 1 1 2 2 H CI CI H 1 1 2 2 1 – high priority 2 – low priority Priority same side – Z Priority opp side - E E/Z naming system ↓ Cahn-Ingold-Prelog (CIP) CH3 H ׀ ׀ C = C ׀ ׀ Br OH 1 – high priority 2 – low priority Priority same side – Z Priority opp side - E 1 2 1 2C atomic mass ↓ Br atomic mass ↑ O atomic mass ↑ H atomic mass ↓ Priority same side - Z
- 4. same atom different atom Cis / Trans naming sys click here E/Z naming system 2 diff substituent on each carbon [Z] 1, 2 dichloroethene [E] 1, 2 dichloroethene CI CI ׀ ׀ C = C ׀ ׀ H H CI H ׀ ׀ C = C ׀ ׀ H CI [Z] 1, 2 dichlorocyclopropane [E] 1, 2 dichlorocyclopropane E / Z naming sys E/Z (CIP priority) 4 diff substituents 1 1 – high priority 2 – low priority 1 2 1 2 2 2 1 Priority same side – Z Priority opp side - E H H CICI 1 1 2 2 H CI CI H 1 1 2 2 1 – high priority 2 – low priority Priority same side – Z Priority opp side - E E/Z naming system Cahn-Ingold-Prelog(CIP) CH3 H ׀ ׀ C = C ׀ ׀ Br OH 1 – high priority 2 – low priority Priority same side – Z Priority opp side - E 1 2 1 2C atomic mass ↓ Br atomic mass ↑ O atomic mass ↑ H atomic mass ↓ Priority same side - Z CI I ׀ ׀ C = C ׀ ׀ H Br CH3 CH3 ׀ ׀ C = C ׀ ׀ H CI [E] 2-chlorobut-2-ene Cis 2 chlorobut-2-ene [Z] 2-bromo-1-chloro-2-iodoethene CI Br ׀ ׀ C = C ׀ ׀ H I [E] 2-bromo-1-chloro-2-iodoethene Br atomic mass ↓ H atomic mass ↓ I atomic mass ↑ 2 2 1 1 CI atomic mass ↑ CI atomic mass ↑ I atomic mass ↑ Br atomic mass ↓ 1 1 2 2 H atomic mass ↓ CH3 CI ׀ ׀ C = C ׀ ׀ H CH3 [Z] 2-chlorobut-2-ene Trans 2 chlorobut-2-ene based on atomic mass 1 1 2 2 1 1 2 2
- 5. CH3 CH3 ׀ ׀ C = C ׀ ׀ H C2H5 Br CH3 ׀ ׀ C = C ׀ ׀ H C2H5 H CH3 ׀ ׀ C = C ׀ ׀ Br C2H5 CH3 H ׀ ׀ C = C ׀ ׀ Br OH 1 1 2 2 1 1 22 E/Z naming system Cahn-Ingold-Prelog(CIP) H COOH ׀ ׀ C = C ׀ ׀ HOOC H 1 – high priority 2 – low priority Priority same side – Z Priority opp side - E 1 1 2 2 H atomic mass ↓ C atomic mass ↑ C atomic mass ↑ H atomic mass ↓ Br H ׀ ׀ C = C ׀ ׀ CH3 OH H atomic mass ↓ C atomic mass ↓ O atomic mass ↑1 1 2 2 Br atomic mass ↑ Br atomic mass ↑ O atomic mass ↑ H atomic mass ↓ 1 2 2 C atomic mass ↓ based on atomic mass 2 11 2 1 1 2 2 1 E - Priority opposite side Z - Priority same side E - Priority opposite side Trans but-2-ene-1,4-dioic acid [Z] 1-bromo-2-methylbut-1-ene Br atomic mass ↑ H atomic mass ↓ H H ׀ ׀ C – C – H ׀ ׀ H H H ׀ C – H ׀ H [E] 1-bromo-2-methylbut-1-ene Br atomic mass ↑ H atomic mass ↓ H H ׀ ׀ C – C – H ׀ ׀ H H C – bond H, H, C (Higher priority) H ׀ C – H ׀ H C – bond H, H, H (Lower priority) [E] 3-methylpent-2-ene C atomic mass ↑ H atomic mass ↓ H H ׀ ׀ C – C – H ׀ ׀ H H C – bond H, H, C (Higher priority) C – bond H, H, C (Higher priority) C – bond H, H, H (Lower priority) C – bond H, H, H (Lower priority) H ׀ C – H ׀ H [Z] 3-methylpent-2-ene H CH3 ׀ ׀ C = C ׀ ׀ CH3 C2H5 H atomic mass ↓ C atomic mass ↑ H H ׀ ׀ C – C – H ׀ ׀ H H H ׀ C – H ׀ H C – bond H, H, C (Higher priority) C – bond H, H, H (Lower priority)
- 6. CH3 H ׀ ׀ C = C ׀ ׀ H C2H5 CH3 CH2OH ׀ ׀ C = C ׀ ׀ CH3CH2 CHO Br CH2OH ׀ ׀ C = C ׀ ׀ CI CHO CI CH2OH ׀ ׀ C = C ׀ ׀ Br CHO CH3 H ׀ ׀ C = C ׀ ׀ Br OH 1 22 1 E/Z naming system Cahn-Ingold-Prelog(CIP) H COOH ׀ ׀ C = C ׀ ׀ HOOC H 1 – high priority 2 – low priority Priority same side – Z Priority opp side - E 1 1 2 2 H atomic mass ↓ C atomic mass ↑ C atomic mass ↑ H atomic mass ↓ Br H ׀ ׀ C = C ׀ ׀ CH3 OH H atomic mass ↓ C atomic mass ↓ O atomic mass ↑1 1 2 2 Br atomic mass ↑ Br atomic mass ↑ O atomic mass ↑ H atomic mass ↓ 1 2 2 C atomic mass ↓ based on atomic mass 2 11 2 1 1 2 2 1 E - Priority opposite side Z - Priority same side E - Priority opposite side Trans but-2-ene-1,4-dioic acid Br atomic mass ↑ CI atomic mass ↓ H ׀ C = O H ׀ C – H ׀ OH Br atomic mass ↑ CI atomic mass ↓ C – bond H, O, O (Higher priority) H ׀ C – OH ׀ H C – bond H, O, O (Higher priority) C – bond H, O, O (Higher priority) C – bond H, O, H (Lower priority) C – bond H, O, H (Lower priority) H ׀ C = O H H ׀ ׀ H – C – C ׀ ׀ H H H ׀ H – C ׀ H C – bond H, H, H (Lower priority) Z - Priority same side H ׀ C = O H ׀ C – H ׀ OH C – bond H, O, H (Lower priority) E - Priority opposite side C – bond H, H, C (Higher priority) Z - Priority same side Pent – 2-ene 2,3-dichlorobut-2-ene H H ׀ ׀ C = C ׀ ׀ CH3 C2H5 [E]-2,3 dichloro but-2-ene CI CI ׀ ׀ C = C ׀ ׀ CH3 CH3 CH3 CI ׀ ׀ C = C ׀ ׀ CI CH3 2 2 2 2 2 22 21 1 1 1 1 1 1 1 [Z] pent-2-ene [E] pent-2-ene [Z]-2,3 dichloro but-2-ene Draw and name isomers using E/Z convention
- 7. Draw structural formula isomer with MF below, state type of isomerism Stereoisomerism C2 H2 CI2 CI CI ׀ ׀ C = C ׀ ׀ H H CI H ׀ ׀ C = C ׀ ׀ CI H Both Structural formula Geometric Isomer CI CI ׀ ׀ C = C ׀ ׀ H H CI H ׀ ׀ C = C ׀ ׀ H CI Cis 1, 2 dichloroethene Trans 1, 2 dichloroethene [Z] 1, 2 dichloroethene [E] 1, 2 dichloroethene 22 11 2 2 1 1 C3H4CI2 CI CI CI CI H HH H Both Structural formula CICICI Geometric Isomer HHH CI H Cis 1, 2 dichlorocyclo propane Trans 1, 2 dichlorocyclo propane [Z] 1, 2 dichlorocyclo propane [E] 1, 2 dichlorocyclo propane Which exhibit cis/transisomerism ? CI H ׀ ׀ C = C ׀ ׀ Br H F CH3 ׀ ׀ C = C ׀ ׀ H CI F CH3 ׀ ׀ C = C ׀ ׀ CI CH3 CH3CH=CHCH3CH3CH=CH2 CH(F)=C(CH3)2 CH(CI)=C(CI)CH3 Draw geometricisomers CH3CH=CHCH2CH3 FCH = CHF Which is structural isomer CH3COCH2CH3 H H ׀ ׀ C = C ׀ ׀ CH3 C2H5 CH3 H ׀ ׀ C = C ׀ ׀ H C2H5 F F ׀ ׀ C = C ׀ ׀ H H F H ׀ ׀ C = C ׀ ׀ H F Which exhibit cis/transisomerism ? CH3CH=CHCH2CH3 FCH = CHF Cis/ [Z] Cis/ [Z]Trans / [E] Trans / [E] CH3CH2-O-CH2CH3CH3CH2CH2-CH ‖ O CH2=CH-CH-CH3 ׀ OH
- 8. Geometric Isomers OpticalIsomers Same chemical property– Same functional gp • Diff physical property – Diff spatial arrangement (Diff density, solubility, melting pt/boiling pt) • Same chemical property – Same functional gp • Same physical property (Same density, solubility, melting pt/boiling pt) Vs Enantiomer Mirror image of each other Enantiomer Mirror image of each other Stereoisomerism Molecules with same molecular formula but diff spatial arrangement • Same molecular formula • Same structural formula /same connectivity • Diff spatial arrangement ofatom Cis Isomer Atom on same side Trans Isomer Atom on diff side click here for optical rotation sugar click here for polarimeter click here opical rotation corn syrupclick here polarimeter Pasco Demo Mirror image Right handed Left handed Non superimposable Chiral/asymmetrical/stereocentre carbon (4 diff groups) same connectivity but diff spatial arrangement
- 9. Isomers with same Molecular Formula and Structural Formula but diff spatial arrangement • At least 1 asymmetric / chiral carbon / stereocentre , bonded to 4 diff gp • NH2CH(R)COOH show optical isomerism • Optical isomers/mirror images call enantiomers (cannot superimpose on each other) • Similar physical and chemical property except for the effect on rotation of plane of polarised light • Optically active – enantiomer rotate plane polarised light to one direction (clockwise / anticlockwise) • Optically inactive – enantiomer present in equal amt (equimolar) – racemic mix and rotation cancel out each other Optical Isomers chiral carbon – 4 diff gp Optically inactive – Rotation cancel out each other Enantiomer (R) - rotate clockwise Enantiomer (S) – rotate anticlock wise 50% 50% 70% 30% Optically active – Net Rotation clockwise Non superimposable Non superimposable
- 10. 1. Light pass through 1st polariser – plane polarised light produced 2. Sample introduce to tube. Sample is optically active Rotate plane of polarised light to one direction 3. Turn analyzereither clockwise/anticlock wise to give light of max intensity again 4. If sample rotate light 120 clockwise – Analyzer need to rotate anticlock wise 120 5. If one enantiomer rotate light 120 clockwise Another enantiomer rotate light anticlock wise 120 How polarimeter detect optical isomer ? 6. Racemic Mix = enantiomers in equal amt (equimolar) , cancel each other rotation 1st polarizer 1st polarizer sample optically active sample optically inactive= Optical activity ability- to rotate plane of polarised light Optically active isomers – presence of asymmetrical/chiral centre - carbon bond to 4 diff gp Product from natural sources/catalysed by enzyme • give 1 pure optically active enantiomer • chiral and found in single enantiomer – optically active Products synthesised chemically • give 2 enantiomer in equal amt /racemic mix • optically inactive rotation cancel out each other Light source 1st polarizer Tube containing sample which able to rotate polarized light 2nd polarizer (Analyzer) Polarizer tube Rotated clockwise How Polarimeter works ? R – inactive Racemate mix ibuprofen S – active Racemate mix ibuprofenIbuprofen (painkiller) Click here notes isomers R limonene S limonene CH3 CH3 CH3
- 11. Product from natural source/catalysed by enzyme • give 1 pure optically active enantiomer • chiral and found in single enantiomer – optically active Product synthesised chemically • give 2 enantiomer in equal amt /racemic mix • optically inactive rotation cancel out each other R – inactive Racemate mix ibuprofen S – active Racemate mix ibuprofen Ibuprofen (painkiller) R limonene S limonene CH3 CH3 CH3 Stereoisomerism Mirror image / enantiomers Same chemical/physical property except rotation of polarized light Source/smell orange Source/smell lemon Mirror image / enantiomers Same chemical/physical property except rotation of polarized light R carvone S carvone Mirror image / enantiomers Same chemical/physical property except rotation of polarized light Source/smell spearmint Source/smell caraway seed R Thalidomide (sedative) S Thalidomide (teratogenic) • Drug company make drug with R and S (racemic mix) • Thalidomide exist as optical isomers • Enantiomers (R) and (S) • (R) effective against morning sickness • S teratogenic, birth and limb defect Our body synthesise enzyme which have active site for only one enantiomer Mirror image / enantiomers Thalidomide (pregnancy) • (S) cause limb defect / shortening of arm /leg • (R) is effective drug • Body convert (R) to (S) by racemisation process, produce racemic mix (R)/(S) • Most drug in racemic mix equal (R) and (S) • Cheaper to synthesise racemic mix than pure enantiomer • Single enantiomer appear to be more effective than racemic mix • Clinical trial is essential to ensure no harmful side effect (S), effective as pain relief (R) has no side effect!
- 12. March 2010 - Publishedin Science by Takumi Ito. Click HERE for info DefectLimb due to Thalidomide • (S) bind /inactivate protein cereblon, which involve in limb formation. • Inactivation,lead to a teratogenic effect on LIMB DEFORMITIES Published in Science ..... • Hiroshi Handa /Takumi Ito - Developed tiny beads with Thalidomide attach. • Thalidomide beads mix with cells extract • Protein Cereblon was bounded on beads • Lack Protein Cereblon during embryo development, cause limb deformities Click HERE for info/source http://www.rsc.org/chemistryworld/news/2010/march/11031001.asp http://news.bbc.co.uk/2/hi/science/nature/8562998.stm http://news.sciencemag.org/sciencenow/2010/03/-thalidomide-ranks-as-one.html Scientist discovered how Thalidomide caused Malformed Limb History of Thalidomide 1954 - Thalidomide for morning sickness. 1961 - Withdrawn, teratogenicity, birth defects result . 1990 - FDA approved for leprosy (skin disease) 1998 - FDA approved for multiple myeloma, (cancer of plasma cells in blood) 2004 - Lenalidomide derived from Thalido able to strengthen immune cells and effective against blood cancers 2011 - Thalidomide + Lenalidomide inhibit formation of new blood vessels ( anti-angiogenic) of tumor cells, Tumors unable to grow due to lack of nutrition. Researchers testing thalidomide in trials for other HIV and Crohn’s disease.
- 13. Write structural formula isomers for C4H9OH, state which isomer show optical isomerism Butan -1-ol Butan-2-ol 2-methylpropan-2-ol 2-methylpropan-1-ol All structuralisomers Stereoisomers (Optical Isomers) Write structural formula of cyclic isomers for C3H4CI2, state type of isomerism Structural formula Geometric Isomers Cis/Tans isomerism Optical Isomers Enantiomer,mirror image Cyclic ring geometric isomers CH3-CH2-CH2-CH3 ׀ OH CH3-CH2-CH-CH3 ׀ OH CH3 ׀ CH3-C-OH ׀ CH3 CH3-CH-CH2-OH ׀ CH3 chiral centre chiral centre CI CI CI CI H HHH H HHH CI CICICI Trans 1, 2 dichlorocyclopropaneCis 1, 2 dichlorocyclopropane Stereoisomers (Optical Isomers) CICI CICI H HHH chiral centre chiral centre * *
- 14. Optical Isomerism Which carbon has chiral center? Draw all stereoisomers CHBr=CHCH(OH)CH3 CHBr=CHCH(OH)CH3 Optical isomersGeometric isomers Chiral carbon with 4 diff gpDouble bond prevent bond rotation Cis / Z Trans / E CH3CH2C* H(CH3)(CI) CH3C* H(NH2)COOH CH3C* H(OH)CH2OH C2H5C* H(OH)CH2OH C2H5 H H ׀ ׀ C = C ׀ ׀ Br CH(OH)CH3 H CH(OH)CH3 ׀ ׀ C = C ׀ ׀ Br H H ׀ CHBr=CH-C–CH3 ׀ OH H ׀ CH3-C-CH=CHBr ׀ OH R (enantiomer) S (enantiomer) chiral centre Non chiral centre NOT mirrorimage superimposable χ rotate it They are same. Superimposable Mirror image Non superimposable chiral centre
- 15. OpticalIsomers Enantiomers Diastereomers Same connectivity Have chiral carbon Non superimposable Mirror image each other Same connectivity Have chiral carbon Non superimposable No Mirror image diff chemical/physicalproperty 2 chiral centre 22 = 4 stereoisomer 3 chiral centre 23 = 8 stereoisomer click here to view diastereomers same chemical/physicalproperty Mirror image Not Mirror image diff configuration at one or more of equivalent stereocentre chiral centre not mirror image same configuration mirror image diff configuration 2, 3 - dibromopentane Diastereomers A B C D Enantiomer/mirror image Enantiomer/mirror image Diastereomer/NOT mirror image Enantiomer/mirror image Diastereomer/NOT mirror image 2n n = chiral centre
- 16. Isomers with same Molecular Formula and Structural Formula but diff spatial arrangement • At least 1 asymmetric / chiral carbon / stereocentre , bonded to 4 diff gp • NH2CH(R)COOH show optical isomerism • Optical isomers/mirror images call enantiomers (cannot superimpose on each other) Optical Isomers chiral carbon – 4 diff gp Non superimposable Non superimposable click here diastereomers OpticalIsomers Enantiomers Diastereomers Same connectivity Have chiral carbon Non superimposable Mirror image each other Same connectivity Have chiral carbon Non superimposable No Mirror image diff chemical/physicalproperty click here diastereomers same chemical/physicalproperty Mirror image Not Mirror image diff configuration at one or more of equivalent stereocentre chiral centre not mirror image same configuration mirror image diff configuration Video on diastereomers
