IB Chemistry on Acid Base Dissociation Constant and Ionic Product Water
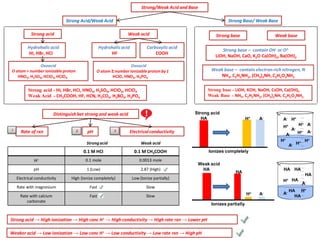
- 1. Strong/Weak Acid and Base Strong Acid/Weak Acid Strong acid - HI, HBr, HCI, HNO3, H2SO4, HCIO3, HCIO4 Weak Acid - CH3COOH, HF, HCN, H2CO3, H3BO3, H3PO4 Strong Base/ Weak Base Strong base - LiOH, KOH, NaOH, CsOH, Ca(OH)2 Weak Base - NH3, C2H5NH2, (CH3)2NH, C3H5O2NH2 Distinguishbet strong and weak acid ElectricalconductivityRate of rxn pH Strongacid Strong acid → High ionization → High conc H+ → High conductivity→ High rate rxn → Lower pH Strong acid Oxoacid O atom > number ionizable proton HNO3, H2SO4, HCIO3,HCIO4 Hydrohalicacid HI, HBr, HCI Weak acid Hydrohalicacid HF Oxoacid O atom ≥ number ionizable protonby 1 HCIO, HNO2, H3PO4 Carboxylicacid COOH Strong base – containOH- or O2- LiOH, NaOH, CaO, K2O Ca(OH)2, Ba(OH)2 Weak base – contain electron rich nitrogen, N NH3, C2H5NH2, (CH3)2NH, C3H5O2NH2 Strong base Weak base 1 2 3 Weak acid 0.1 M HCI 0.1 M CH3COOH H+ 0.1 mole 0.0013 mole pH 1 (Low) 2.87 (High) Electrical conductivity High (Ionize completely) Low (Ionize partially) Rate with magnesium Fast Slow Rate with calcium carbonate Fast Slow Weaker acid → Low ionization → Low conc H+ → Low conductivity→ Low rate rxn → High pH Strong acid HA A-H+ H+ H+ H+ H+ H+ H+ H+A- A- A- A- A- A- Ionizes completely Weak acid HA HA H+ A- H+ H+ A- A- HA HA HA HA HA HA Ionizes partially
- 2. Easier using pH scale than Conc [H+] • Conc H+ increase 10x from 0.0001(10-4) to 0.001(10-3) - pH change by 1 unit from pH 4 to 3 • pH 3 is (10x) more acidic than pH 4 • 1 unit change in pH is 10 fold change in Conc [H+] Conc OH- increase ↑ by 10x pH increase ↑ by 1 unit pOH with Conc OH- pOH = -log [OH- ] [OH- ] = 0.0000001M pOH = -log [0.0000001] pOH = -log1010-7 pOH = 7 pH + pOH = 14 pH + 7 = 14 pH = 7 (Neutral) pH with Conc H+ pH = -log [H+ ] [H+ ] = 0.0000001M pH = -log [0.0000001] pH = -log1010-7 pH = 7 (Neutral) Conc H+ increase ↑ by 10x pH decrease ↓ by 1 unit pH measurement of Acidity of solution • pH is the measureof acidity of solutionin logarithmicscale • pH = powerof hydrogenor minuslogarithmto base ten of hydrogenion concentration ← Acidic – pH < 7 Alkaline – pH > 7 → pOH with Conc OH- pOH = -log [OH- ] [OH- ] = 0.1M pOH = -log[0.1] pOH = 1 pH + pOH = 14 pH + 1 = 14 pH = 13 (Alkaline) pH with Conc H+ pH = -log [H+ ] [H+ ] = 0.01M pH = -log [0.01] pH = -log1010-2 pH = 2 (Acidic) Easier pH scaleConc H+
- 3. Conc [H+ ] = 1 x 10-12 pH = -lg[H+ ] pH = -lg[10-12 ] pH = 12 Conc [OH- ]= 1 x 10-2 pOH = -log10[OH-] pOH = -log1010-2 = pOH = 2 pH + pOH = 14 pH + 2 = 14 pH = 12 Conc [H+ ] = 1 x 10-2 pH = -lg[H+ ] pH = -lg[10-2 ] pH = 2 Alkaline Alkaline Acidic Acidic Kw - Ionic product constant water Using conc [H+] pH = -log10[H+] pH = -log10[H+] pOH = -log10[OH-] pH + pOH = 14 Kw = [H+][OH-] Using conc [OH-] pOH = -log10[OH-] Conc [OH- ]= 1 x 10-12 pOH = -log10[OH-] pOH= -log1010-12 =pOH = 12 pH + pOH = 14 pH + 12 = 14 pH = 2 Formula for acid/basecalculation OH OHOH Kc 2 3 OHOHOHKc 32 OHOHKw 3 OHOH3 14 100.1 7714 101101100.1 7 101 OH OHOHOHOH 322 H2O dissociateforming H3O+ and OH- (equilibriumexist) 14 100.1 wK Dissociation water small [H2O] is constant Kw = 1.0 x 10-14 Ionic Product constantwater at -25C Kc - Dissociation constant water 7 3 101 OH
- 4. Number sig fig in log calculation Significant number in log calculation log10(3575)=3.55327 = 3.5532 log10(3.000x104) = 4.477121 = 4.4771 log10(3.3 x 104) = 4.5185 = 4.51 Calculation involve pH = -log10[H+] Conc H+ = 1.9 x 10-4 pH= -log10[1.9 x 10-4] = 3.721 = 3.72 Measurement scale not linear • Simple average CANNOT be used • Average of pH 7, pH 8, pH 9 pH scale is logarithmic, pH = -log[H+] Correct average = convert to H+ conc pH 7 = -log10[H+] → H+ = 10-7 pH 8 = -log10[H+] → H+ = 10-8 pH 9 = -log10[H+] → H+ = 10-9 pH pH= -lg10H+ Conc H+ 0 0 = -lg10100 1.0 1 1 = -lg1010-1 0.1 2 2 = -lg1010-2 0.01 3 3 = -lg1010-3 0.001 4 4 = -lg1010-4 0.0001 5 5 = -lg1010-5 0.00001 6 6 = -lg1010-6 0.000001 7 7 = -lg1010-7 0.0000001 8 8 = -lg1010-8 0.00000001 9 9 = -lg1010-9 0.000000001 10 10= -lg1010-10 0.0000000001 11 11= -lg1010-11 0.00000000001 12 12= -lg1010-12 0.000000000001 13 13= -lg1010-13 0.0000000000001 14 14= -lg1010-14 0.00000000000001 Easier using pH scale than Conc [H+] • Low pH – High H+ conc – More acidic • High pH – Low H+ conc – Less acidic • pH 3 (10x) more acidic > than pH 4 • 1 unit change in pH is 10 fold change in Conc [H+] Relationship between pH and Conc H+ Uncertainty involving pH 8 3 987 Average Uncertainty involving pH 4 sig fig 5 sig fig/4 decimal place 4 sig fig 5 sig fig/4 decimal place Conc H+ = 3.2 x 10-5 M pH = - log10[3.2 x 10-5]= 4.4948 = 4.49 2 sig fig 3 sig fig/2 decimal place 2 sig fig3 sig fig/2 decimal place 2 sig fig3 sig fig/2 decimal place 2 sig fig 3 sig fig 2 sig fig3 sig fig 2 sig fig 3 sig fig pH solution = 7.40. Cal conc of H+ ions 7.40 = -log10 [H+] [H+] = 10-7.40 = 4.0 x 10-8 3 sig fig 2 sig fig 2 sig fig 4.7 ]107.3lg[ 107.3 3 101010 8 8 987 pH pH Average Average
- 5. pH weak acid at variousconcentration OHCOOCHOHCOOHCH 3323 Extendof dissociationdependon initialconcentrationacid Conc of acid Observed pH CH3COOH CalculatedpH HCI 0.10 2.7 1.0 0.010 3.0 2.0 0.0010 3.5 3.0 0.00010 4.2 4.0 CIHHCI Weak acid Strong acid Dissociate partially Dissociate completely At same acid concentration • HCI has HIGHER[H+] > CH3COOH • HCI has LOWER pH < CH3COOH • HCI dissociate completely- Strong acid • CH3COOH dissociatepartially- Weak acid At decreasing acid concentration • Extend of dissociation for CH3COOH increase • pH weak acid closer to strong acid • Dilution increase the extend of dissociation Conc decrease OHCOOCHOHCOOHCH 3323 Trends Addition Water Dilution shift equilibrium to right Decreaseconc of CH3COOH,CH3COO- andH+ Conc on left side is more effecteddue to CH3COO- and H+ Equilibrium shift to right to increase conc of CH3COO- andH+ again Extend of dissociation for acid increase (shift to right) О О Concept Map [H+] [OH-] pH pOH Kw = [H+] x [OH-] = 1 x 10-14 pH + pOH = 14 pH = -lg [H+] [H+] = 10-pH pOH = -lg [OH-] [OH-] = 10-pOH
- 6. OHOH3 14 100.1 7714 101101100.1 OHOHOHOH 322 H2O dissociate forming H3O+ and OH- (equilibrium exist) 14 100.1 wK Kw = 1.0 x 10-14 Ionic Product constant water at -25C Kc – Ionic Product Constant Water H+ OH- Ionic Product Water, Kw, is Temperature dependent Temp/C Kw [H+] [OH-] pH 0 1.5 x 10-15 0.39 x 10-7 0.39 x 10-7 7.47 10 3.0 x 10-15 0.55 x 10-7 0.55 x 10-7 7.27 20 6.8 x 10-15 0.82 x 10-7 0.82 x 10-7 7.08 25 1.0 x 10-14 1.00 x 10-7 1.00 x 10-7 7.00 30 1.5 x 10-14 1.22 x 10-7 1.22 x 10-7 6.92 40 3.0 x 10-14 1.73 x 10-7 1.73 x 10-7 6.77 50 5.5 x 10-14 2.35 x 10-7 2.35 x 10-7 6.63 OHOHOHOH 322 OHOHKw 3 molkJH /57 Temp increase↑ → Equilibrium shift right → Reduce Temp ↓ → More ion form Kw increase↑ Temp ↑ - shift right – more H+ /OH- – Kw ↑ Temp ↑ - Kw ↑ – H+ ion ↑ - pH ↓ At 25C, Kw - 1.0 x 10-14 Conc [H+] = [OH−]= 1.0 x 10-7 Neutral pH = 7 At 50C, Kw - 5.5 x 10-14 Conc [H+]= [OH−]= 2.35 x 10-7 Neutral pH = 6.63 OHOHKw 3 At 25C, Kw - 1.0 x 10-14 •Kw = [H+][OH−] • 1.0 x 10-14 = [H+][OH−] • [H+][OH−] = [10-7][10-7] • pH = -lg[H+ ] • pH = -lg [1.0 x 10-7] • Neutral pH = 7 At 50C, Kw - 9.3 x 10-14 •Kw = [H+][OH−] •9.3 x 10-14 = [H+][OH−] •[H+]2 = 9.3 x 10-14 •[H+] = 3.05 x 10-7 • pH = -lg[3.05 x 10-7] • Neutral pH = 6.5 Amount same Amount same
- 7. Ionic Product Water, Kw, is Temperature dependent Temp/ C Kw [H+] [OH-] pH 0 1.5 x 10-15 0.39 x 10-7 0.39 x 10-7 7.47 10 3.0 x 10-15 0.55 x 10-7 0.55 x 10-7 7.27 20 6.8 x 10-15 0.82 x 10-7 0.82 x 10-7 7.08 25 1.0 x 10-14 1.00 x 10-7 1.00 x 10-7 7.00 30 1.5 x 10-14 1.22 x 10-7 1.22 x 10-7 6.92 40 3.0 x 10-14 1.73 x 10-7 1.73 x 10-7 6.77 50 5.5 x 10-14 2.35 x 10-7 2.35 x 10-7 6.63 OHOHOHOH 322 OHOHKw 3 molkJH /57 Temp increase↑→ Equilibrium shift right → Reduce Temp ↓→ More ion form Kw increase ↑ Temp ↑ - shift right – more H+ /OH- – Kw ↑ Temp ↑ - Kw ↑ – H+ ion ↑ - pH ↓ At 25C, Kw - 1.0 x 10-14 Conc [H+] = [OH−]= 1.0 x 10-7 Neutral pH = 7 At 50C, Kw - 5.5 x 10-14 Conc [H+]= [OH−]= 2.35 x 10-7 Neutral pH = 6.63 Kc ionization water = 1.80 x 10-16. Based on magnitude of Kc which direction does it lies? Calculate Kw for water assume [H2O] is constant = 55.6 mol/dm3 OH OHH Kc 2 Kw = 1.0 x 10-14 Ionic Product constant water at -25C • Direction to the left • Mostly undissociated water molecules treac product Kc tan 14 16 16 100.1 1080.155 55 1080.1 OHH OHH OHH 14 100.1 wK OHHOH2 OHHOHKK cw 2 Fraction of ionized = Amt ionized = 1.00 x 10-7 = 18 x 10-10 Initial amt 55.6 7714 101101100.1 Kc small 18 molecule ionized in 10 000 000 000 Amount same Amount same
- 8. Formula for acid/basecalculation [OH-][H+] Kw = [H+] x [OH-] = 1 x 10-14 [OH-] = 10-pOHpOH = -lg [OH-] pOHpH pH = -lg [H+] [H+] = 10-pH pH + pOH = 14 Formula for acid/basecalculation DissociationConstant for Weak Acid pH = -log10[H+] pOH = -log10[OH-] pH + pOH = 14 pH + pOH = pKw Kw = [H+][OH-] Ka x Kb = Kw Ka x Kb = 1 x 10-14 pKa = - lg10Ka pKb = - lg10Kb pKa + pKb = pKw pKa + pKb = 14 AHHA HA AH Ka HCOOCHCOOHCH 33 COOHCH H COOHCH HCOOCH Ka 3 2 3 3 DissociationConstant for Weak Base OHBHOHB 2 B OHBH Kb OHNHOHNH 423 3 2 3 4 NH OH NH OHNH Kb OHCOOCHOHCOOHCH 3323 OHCOOCHOHCOOHCH 3323 COOHCH OHCOOCH Ka 3 33 OHCOOHCHOHCOOCH 323 COOCH OHCOOHCH Kb 3 3 Derive Ka x Kb = Kw Relationship bet Weak acid and its conjugate base Weak acid Conjugate Base COOCH OHCOOHCH COOHCH OHCOOCH 3 3 3 33 OHOH COOCH OHCOOHCH COOHCH OHCOOCH 3 3 3 3 33 wba KKK
- 9. Formula for acid/basecalculation Ka /Kb measureequilibriumposition Ka/Kb large ↑ – ↑ dissociation– shift to right – favour product Ka/Kb large ↑ – pKa /pKb small ↓ – Strongeracid/base Strongacid Large ↑ Ka Weak acid Small ↓ Ka Strongbase Large ↑ Kb Weak base Small ↓Kb ↑ Ka → ↓ pKa Ka /Kb measureequilibriumposition Ka /Kb small ↓ – ↓ dissociation– shift to left – reactant favour Ka /Kb small ↓ – pKa /pKb high ↑– Weak acid/base ↑ Kb → ↓ pKb ↓ Ka → ↑ pKa ↓ Kb →↑ pKb For weak acid/ base CIHHCI OHNHOHNH 423 Shift right Shift left CH3COOH + H2O ↔ CH3COO- + H3O+ CH3COOH CH3COO-CH3COOH ↔ CH3COO- Strong Acid Weak conjugate BaseConjugate acid base pair Small dissociation constant Strong Acid Weak base ba KK / Strongacid Strongbase
- 10. Formula for acid/basecalculation [OH-][H+] Kw = [H+] x [OH-] = 1 x 10-14 [OH-] = 10-pOHpOH = -lg [OH-] pOHpH pH = -lg [H+] [H+] = 10-pH pH + pOH = 14 Formula for acid/basecalculation DissociationConstant for Weak Acid pH = -log10[H+] pOH = -log10[OH-] pH + pOH = 14 pH + pOH = pKw Kw = [H+][OH-] Ka x Kb = Kw Ka x Kb = 1 x 10-14 pKa = - lg10Ka pKb = - lg10Kb pKa + pKb = pKw pKa + pKb = 14 AHHA HA AH Ka HCOOCHCOOHCH 33 COOHCH H COOHCH HCOOCH Ka 3 2 3 3 DissociationConstant for Weak Base OHBHOHB 2 B OHBH Kb OHNHOHNH 423 3 2 3 4 NH OH NH OHNH Kb Dissociatepartially ↔ used Weak acid/base Ka /Kb value pKa /pKb value easier! Click here weak acid dissociation Click here weak acid dissociation Click here CH3COOH dissociation Click here strong acid ionization Weak acid/base Animation
- 11. What is pH for [H+ ] = 1 x 10-12 M pH = -lg [10-12 ] pH = 12 What is conc of H+ of pH 3.20? 3.20 = -lg [H+ ] [H+ ] = 10 –2.20 [H+ ] = 6.3 x 10-4 pH = -log10[H+] pOH = -log10[OH-] pH + pOH = 14 Kw = [H+][OH-] Formulaacid/basecalculation 2 sig fig 1 sig fig 3 sig fig 2 sig fig What is pH for [OH- ] = 0.15M pOH = -lg [0.15] pOH = 0.823 pH + pOH = 14 pH = 14 – 0.823 = 13.2 pOH = -log[OH-] 3 sig fig 2 sig fig Calculate conc of H+, OH- and pH for 0.001 M HCI. 1 2 3 4 CIHHCI 0.001 ↔ 0.001 0.001 OHHOH2 HCIH2O OHHKw Assuming H+ all from HCI = 0.0010 )()( 2OHHHCIHH = 0.001 Negligible / too little OHH14 100.1 0.3 001.0log log 10 10 pH pH HpH 0.31114 11 101 001.0 100.1 001.0100.1 11 14 14 pH pOH OH OH or Cal conc OH- /pH when3.o x 10-4 H+ add water HCI H2O CIHHCI OHHOH2 OHHKw OHH14 100.1 11 4 14 414 103.3 100.3 100.1 100.3100.1 OH OH 3x10-4 ↔ 3x10-4 52.3 100.3log log 4 10 10 pH pH HpH 5
- 12. 11 10loglog 101 001.0 100.1 001.0100.1 11 11 14 14 pH HpH H H 00.1 10.0log log 10 10 pH pH HpH Cal pH of 0.10 M HCI H2O Assuming H+ all from HCI = 0.10 )()( 2OHHHCIHH CIHHCI 0.10 mol 0.10 mol = 0.10 StrongAcid/Base calculation Strong acid • 100% dissociation (complete) Strongbase • 100% dissociation (complete) CIHHCI OHKKOHShift right Shift right 2 sig fig3 sig fig Cal pH of 0.10M H2SO4 H2O 2 442 2 SOHSOH 0.10 mol 0.20 mol Assuming H+ all from H2SO4 = 0.20 700.0 20.0log log 10 10 pH pH HpH )()( 242 OHHSOHHH = 0.20 2 sig fig3 sig fig OHKKOH 0.001 mol 0.001 mol Cal pH of 0.001 M KOH H2O Assume OH- from KOH = 0.10 )()( 2OHOHKOHOHOH OHHKw = 0.001 11,3 001.0log log pHpOH pOH OHpOH OHCaOHCa 2)( 2 2 0.001 mol 0.002 mol Cal pH of 0.001M Ca(OH)2 H2O Assume OH- from Ca(OH)2 = 0.002 )()( 2OHOHKOHOHOH = 0.002 3.11,7.2 002.0log log pHpOH pOH OHpOH OHHKw 3.11 105log 105 002.0 100.1 002.0100.1 12 12 14 14 pH pH H H
- 13. 0.2 01.0log log 10 10 pH pH HpH OH OHOH Kc 2 3 OHOHKOHK wc 32 OHOH3 14 100.1 7714 101101100.1 OHOHOHOH 322 H2O dissociate forming H3O+ and OH- (equilibrium exist) 14 100.1 wK Dissociation water small [H2O] is constant Kw - Ionic product constant water Kw = 1.0 x 10-14 Ionic Product constant water at -25C Kc - Dissociation constant water Cal conc of H+ ,OH- and pH of water Cal conc of H+ ,OH- and pH of 0.01M HCI OHHOH2 OHHKw OHH14 100.1 7714 101101100.1 7 101 H H2O H2O HCI OHHOH2 H2O OHHKw OHH14 100.1 Assuming H+ all from HCI = 0.01 )()( 2OHHHCIHH H+ = 0.01 + 1.0x10-12 = 0.01 + 0.000000000001 ≈ 0.01 OHHOH2 H+ = 1x10-12 OH- = 1x10-12 CIHHCI 0.01 mol 0.01 mol0.01 1 mol ↔ 1 mol 1mol 0.000000000001 0.000000000001 H+ OH- = 0.01 = 0.000000000001 or 0.7 101log 7 10 pH pH 0.21214 12 100.1 01.0 100.1 01.0100.1 12 14 14 pH pOH OH OH
- 14. OH OHOH Kc 2 3 OHOHKOHK wc 32 OHOH3 14 100.1 7714 101101100.1 OHOHOHOH 322 H2O dissociate forming H3O+ and OH- (equilibrium exist) 14 100.1 wK Dissociation water small [H2O] is constant Kw - Ionic product constant water Kw = 1.0 x 10-14 Ionic Product constant water at -25C Kc - Dissociation constant water Cal conc of H+ ,OH- and pH of 0.01M KOH Cal conc of H+ ,OH- and pH of 0.1M H2SO4 7.0 2.0log log 10 10 pH pH HpH H2O KOH H2SO4 OHHOH2 H2O OHHKw OHH14 100.1 Assuming H+ all from H2SO4 = 0.2 7.03.1314 3.13 100.5 2.0 100.1 2.0100.1 14 14 14 pH pOH OH OH )()( 242 OHHSOHHH H+ = 0.2 + 5 x 10-14 = 0.2 + 0.000000000000005 ≈ 0.2 OHHOH2 H+ = 5x10-14 OH- = 5x10-14 2 442 2 SOHSOH 0.1 mol 0.2 mol 0.2 1 mol ↔ 1 mol 1 mol 0.00000000000005 0.0000000000005 H+ OH- = 0.2 = 0.00000000000005 OHHOH2 1 mol ↔ 1 mol 1 mol OHHOH2 OHHKw OHKKOH 0.01 Assuming OH- all from KOH = 0.01 )()( 2OHOHKOHOHOH = 0.01 = 0.000000000001 12 10log log 101 01.0 100.1 01.0100.1 12 10 10 12 14 14 pH pH HpH H H or H+ = 1x10-12 OH- = 1x10-12 0.01 mol 0.01 mol
- 15. Approximationand Assumption Ka very small < 10-5 Not much change acid conc Approximation is VALID Ionizationmake no diff to conc HA SMALLKa SMALLKa Find pH of 0.10 M HA, weak acid Ka - 1.8 x 10-5 - Little product form - Initial conc reactant unchanged Using approximation 0.10 – x ≈ 0.10 HA ↔ H+ + A- Initial conc 0.10 0 0 Change 0.10 - x +x +x Eq Conc 0.10 – x +x +x HA ↔ H+ + A- HA AH Ka x x 10.0 108.1 2 5 10.0 108.1 2 5 x 3 1034.1 x [HA] = (0.10 – 0.00134) = 0.098 ≈ 0.10 [HA]initial ≈ [HA]eq CalculationWeak Acid (UsingICE Method) 87.2 1034.1log log 3 10 10 pH pH HpH HA ↔ H+ + A- Find Ka of 0.02 M HA, weak acid, [H]+ = 0.0012M HA ↔ H+ + A- Initial conc 0.02 0 0 Change 0.02 – 0.0012 +0.0012 +0.0012 Eq Conc ≈ 0.02 +0.0012 +0.0012 HA AH Ka 02.0 0012.00012.0 aK Using approximation 0.02 – 0.0012 ≈ 0.02 5 102.7 aK Find Ka of 0.01M HA, weak acid, pH = 5.0 HA ↔ H+ + A- HA ↔ H+ + A- Initial conc 0.01 0 0 Change 0.01 – 1x10-5 +1x10-5 +1x10-5 Eq Conc ≈ 0.01 +1x10-5 +1x10-5 5 10 101 log0.5 H H HA AH Ka 01.0 101101 55 aK Using approximation 0.01 – 1x10-5 ≈ 0.01 8 108.1 aK < 5% rule %3.1%100 10.0 1034.1 %5 3 concInitial x
- 16. Approximation and Assumption SMALLKa SMALLKa HA ↔ H+ + A- HA AH Ka HA H 2 6 101.4 4 104.2 HA CalculationWeak Acid (UsingICE Method) 5 101.3 log50.4 log H H HpH Find pH of 0.100M HA, weak acid, pKa = 4.20 HA AH Ka 600.2 1051.2log 3 pH pH Find Conc HA, weak acid, pH = 4.50, Ka = 4.1 x 10-6 HA 25 6 101.3 101.4 HA ↔ H+ + A- 100.0 1031.6 2 5 H 5 1031.6 log2.4 log a a aa K K KpK 3 1051.2 H 3 sig fig4 sig fig Find Ka of 0.01M CH3COOH,pH = 3.4 CH3COOH ↔ CH3COO- + H+ Initial conc 0.01 0 0 Change 0.01 – 0.0004 +0.0004 +0.0004 Eq Conc ≈ 0.01 +0.0004 +0.0004 0004.001.0 104 24 3 2 3 3 COOHCH H COOHCH HCOOCH Ka 4 104 log4.3 log H H HpH 5 106.1 aK 01.0 104 24 aK Using approximation 0.01 – 0.0004 ≈ 0.01 Ka very small < 10-5 Not much change acid conc Approximation VALID Ionization make no diff to conc acid Find pH of 0.75M CH3COOH,Ka = 1.8 x 10 -5 CH3COOH ↔ CH3COO- + H+ Initial conc 0.75 0 0 Change 0.75 - x +x +x Eq Conc ≈ 0.75 +x +x x x COOHCH HCOOCH Ka 75.0 2 3 3 Using approximation 0.75 – x ≈ 0.75 75.0 108.1 2 5 H 3 107.3 H 40.2 107.3log 3 pH pH
- 17. Approximationand Assumption Kb very small < 10-5 Not much change reactant conc Approximation is VALID Ionizationmake no diff to conc B SMALLKb SMALLKb Find pH of 0.010M B, weak base Kb - 1.8 x 10-5 - Little product form - Initial conc reactant unchanged Using approximation 0.01 – x ≈ 0.01 B + H2O ↔ BH+ + OH- Initial conc 0.01 0 0 Change 0.01 - x +x +x Eq Conc 0.01 – x +x +x B + H2O ↔ BH+ + OH- B OHBH Kb x x 010.0 108.1 2 5 4 102.4 x [B] = (0.01 – 0.00042) ≈ 0.01 [B]initial ≈ [B]eq CalculationWeak Base (Using ICE Method) 6.10 37.314 37.3 102.4log log 4 pH pH pOH pOH OHpOH Find Kb of 0.030M B, weak base, pH = 10.0 Using approximation 0.03 – 0.0001 ≈ 0.03 7 103.3 bK 010.0 108.1 2 5 x B + H2O ↔ BH+ + OH- B + H2O ↔ BH+ + OH- Initial conc 0.03 0 0 Change 0.03 - x +x +x Eq Conc 0.03 – x +x +x B OHBH Kb x x Kb 030.0 2 4 100.1 log4 log 1014 OH OH OHpOH pOH 030.0 100.1 24 bK Find conc of B, weak base, pH 10.8, Kb - 4.36 x 10-4 B + H2O ↔ BH+ + OH- B OHBH Kb B 22.3 4 100.1 1036.4 2.3 100.1 log2.3 log 8.1014 OH OH OHpOH pOH 4 101.9 B < 5% rule %2.4%100 01.0 102.4 %5 4 concInitial x
- 18. x x NH OH 20.0 2 3 2 20.0 108.1 2 5 x Approximationand Assumption SMALLKb SMALLKb CalculationWeak Base (Using ICE Method) 60.11400.214 400.2 1046.4log log 3 pH pOH pOH OHpOH 3 1046.4 OH 3 4 NH OHNH Kb 3 109.1 x Using approximation 0.20 – 0.0019 ≈ 0.20 4 101.9 B Find pH of 0.0500M B, weak base pKb - 3.40 B + H2O ↔ BH+ + OH- B OHBH Kb 0500.0 1098.3 2 4 OH 4 1098.3 log40.3 log b b bb K K KpK Find conc of B, weak base pH - 10.8, pKa = 10.64 B + H2O ↔ BH+ + OH- B OHBH Kb B OH 2 4 1036.4 4 1036.4 log36.3 log 36.3 64.1014 14 b b bb b b ba K K KpK pK pK pKpK 4 103.6 log2.3 log 2.38.1014 OH OH OHpOH pOH B 24 4 103.6 1036.4 NH3 + H2O ↔ NH4 + + OH- Initial conc 0.20 0 0 Change 0.20 – x +x +x Eq Conc ≈ 0.20 +x +x Find pH of 0.20M NH3 - Kb - 1.8 x 10-5 28.11 72.214 72.2 109.1log log 3 pH pH pOH pOH OHpOH Find Kb of 0.10M CH3NH2 , pH = 11.8 CH3NH2 + H2O ↔ CH3NH3 + + OH- Initial conc 0.10 0 0 Change 0.10 – x +x +x Eq Conc 0.10 - x +x +x 23 33 NHCH OHNHCH Kb x x 10.0 2 3 103.6 log20.2 20.28.1114 14 OH OH pOH pOHpH 3 3 103.610.0 103.6 bK 4 102.4 bK > 5% rule Approximation %7.6%100 10.0 103.6 %5 3 concInitial x
- 19. H+ = 1 x 10-8 + 9.5 x 10-8 H+ = 10.5 x 10-8 980.6 105.10log log 8 10 10 pH pH HpH H2O HCI OHHOH2 H2O OHHKw xx 814 101100.1 Assuming H+ from HCI and H2O )()( 2OHHHCIHH OHHOH2 CIHHCI x H+ = 1 x 10-8 + x 8 105.9 x Cal pH of 0.10M HCI CIHHCI 0.10 mol 0.10 mol Assuming H+ all from HCI = 0.10 )()( 2OHHHCIHH = 0.10 00.1 10.0log log 10 10 pH pH HpH 2 sig fig3 sig fig Cal pH of 1 x 10-8M HCI 1 x 10-8Assuming dissociation H+ ions from water = x 1 x 10-8 pH of very STRONG CONC acid Strong acid • 100% dissociation (complete) CIHHCI Shift right H+ ions from water is negligible Assume all H+ ions come from ACID pH of very STRONG DILUTED acid H+ ions from water is SIGNIFICANT All H+ ions come from ACID and H2O Assuming H+ from HCI = 1 x 10-8 0.8 101log log 8 10 10 pH pH HpH ALKALINE!!!!!!! Click here to view Table for Ka/KbExpt acid/base (RSC) Click here to view 1 x 10-8
- 20. Formula for acid/base calculation [OH-][H+] Kw = [H+] x [OH-] = 1 x 10-14 [OH-] = 10-pOHpOH = -lg [OH-] pOHpH pH = -lg [H+] [H+] = 10-pH pH + pOH = 14 pH = -log10[H+] pOH = -log10[OH-] pH + pOH = 14 pH + pOH = pKw Kw = [H+][OH-] Ka x Kb = Kw Ka x Kb = 1 x 10-14 pKa = - lg10Ka pKb = - lg10Kb pKa + pKb = pKw pKa + pKb = 14 HF + H2O ↔ F- + H3O+ STRONG BASE WEAK BASE Kb increase pKb increasepKb decrease Kb decrease STRONG ACID WEAK ACID Ka increase pKa decrease Ka decrease pKa increase Ka pKa Kb pKb pKa = -lg [Ka] pKb = -lg [Kb] Ka = 10-pKa Kb = 10-pKb Ka x Kb = Kw Ka x Kb = 1 x 10-14 pKa + pKb = 14 pKa + pKb = pKw Find Kb for F- , Ka HF - 6.8 x 10-4 HF (acid) - F- (conjugate base) 4 4 14 14 1098.3 108.6 101 101 )()( b b a b wba K K K K KFKHFK 3 4 2 4 2 442 4243 POHHPO HPOHPOH POHHPOH Successive acid dissociation constant 3 443 3 POHPOH Polyprotic acid – dissociate releasing 1 proton each time 13 3 8 2 3 1 108.4 102.6 105.7 K K K 3 443 3 POHPOH + Less acidic Increasing difficulty removing H+ from negatively charged ion Most acidic wba KKK
- 21. Click here on pH calculation Video on Acid/ Base Click here on pKa /pKb calculation How pH = pOH = 14 derived How Ka x Kb = Kw derived Simulation on Acid/ Base Click here on pH animation Click here to acid/base simulation Click here on weak base simulation Click here strong acid ionization Click here on weak acid dissociation
