Lecture 19.2- pH
•
2 likes•1,234 views
Section 19.2 Lecture for Honors & Prep Chemistry
Report
Share
Report
Share
Recommended
More Related Content
Viewers also liked
Viewers also liked (6)
Similar to Lecture 19.2- pH
Similar to Lecture 19.2- pH (20)
IB Chemistry on Acid Base Dissociation Constant and Ionic Product Water

IB Chemistry on Acid Base Dissociation Constant and Ionic Product Water
More from Mary Beth Smith
More from Mary Beth Smith (20)
Chapter 3 and 5 lecture- Ecology & Population Growth

Chapter 3 and 5 lecture- Ecology & Population Growth
Biotechnology Chapter Five Lecture- Proteins (part b)

Biotechnology Chapter Five Lecture- Proteins (part b)
Biotechnology Chapter Five Lecture- Proteins (part a)

Biotechnology Chapter Five Lecture- Proteins (part a)
Recently uploaded
Recently uploaded (20)
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx

HMCS Max Bernays Pre-Deployment Brief (May 2024).pptx
UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf

UGC NET Paper 1 Mathematical Reasoning & Aptitude.pdf
Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...

Jual Obat Aborsi Hongkong ( Asli No.1 ) 085657271886 Obat Penggugur Kandungan...
Fostering Friendships - Enhancing Social Bonds in the Classroom

Fostering Friendships - Enhancing Social Bonds in the Classroom
Food safety_Challenges food safety laboratories_.pdf

Food safety_Challenges food safety laboratories_.pdf
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
Salient Features of India constitution especially power and functions

Salient Features of India constitution especially power and functions
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
Vishram Singh - Textbook of Anatomy Upper Limb and Thorax.. Volume 1 (1).pdf

Vishram Singh - Textbook of Anatomy Upper Limb and Thorax.. Volume 1 (1).pdf
Lecture 19.2- pH
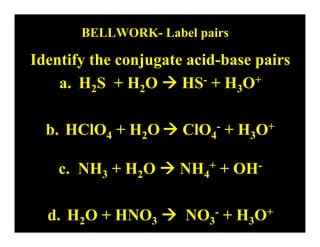
- 1. BELLWORK- Label pairs Identify the conjugate acid-base pairs a. H2S + H2O HS- + H3O+ b. HClO4 + H2O ClO4- + H3O+ c. NH3 + H2O NH4+ + OH- d. H2O + HNO3 NO3- + H3O+
- 2. The pH scale measures the hydrogen ion concentration[H+] of a solution.
- 3. A pH of 7 is neutral A pH less than 7 is acidic (litmus is red) A pH greater than 7 is basic (litmus is blue) The pH scale ranges from below zero (very acidic) to above 14 (very basic)
- 4. The pH scale is not linear. The pH scale is logarithmic. pH = -log[H+] [H+] = 1.0 x 10-2 pH = 2 very acidic [H+] = 1.0 x 10-3 pH = 3 acidic A solution with pH of 2 contains 10 times as much H+ as a solution with pH of 3.
- 5. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M [H+] = 5.0 x 10-5M
- 6. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M pH= -(exponent) =7 [H+] = 5.0 x 10-5M
- 7. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M pH= -(exponent) =7 [H+] = 5.0 x 10-5M pH is between 4&5
- 8. Calculate pH pH = -log [H+] [H+] = 1.0 x 10-7M pH= -(exponent) =7 [H+] = 5.0 x 10-5M pH is between 4&5 to CHECK with calculator 1.Enter concentration in calculator 2.Press log key 3.Change sign to positive OR in the reverse order depending on your calculator!
- 9. Calculate [H+] given pH pH = 7.0 pH= 8.5
- 10. Calculate [H+] given pH pH = 7.0 [H+]= 1x10-7 pH= 8.5
- 11. Calculate [H+] given pH pH = 7.0 [H+]= 1x10-7 pH= 8.5 [H+]= 3.2x10-9
- 12. Calculate [H+] given pH pH = 7.0 [H+]= 1x10-7 pH= 8.5 [H+]= 3.2x10-9 = between 1x10-9 and 1x10-8 1. Enter pH value in calculator 2. Press the +/- key 3. Press the 10x key OR in the reverse order depending on your calculator!
- 13. From pH0 to pH14 the H+ concentration decreases 100,000,000,000,000 times!!
- 14. Relationship between [H+] and [OH-] [H3O+][OH-] = 1.0 x 10-14 ALWAYS!!
- 15. Relationship between [H+] and [OH-] [H3O+][OH-] = 1.0 x 10-14 ALWAYS!! If [H+] = 1.0 x 10-5 then, [OH-] = 1.0x10-14/ 1.0x10-5 = 1 x 10-9 SUBTRACT THE EXPONENTS!
- 16. Relationship between [H+] and [OH-] [H3O+][OH-] = 1.0 x 10-14 If [H+] = 5.0 x 10-4 then, [OH-] = 1.0x10-14/ 5.0x10-4 = 2 x 10-11
- 17. Acidic = more H+ than OH- Basic = more OH- than H+ There is always some of each ion in water no matter what the pH
- 19. Relationship between pH and pOH pOH = -log[OH-] pH + pOH = 14 Always!!
- 20. pH pH + pOH = 14 pOH pH = pOH = -log[H+] -log[OH-] [H+] [H+][OH-]=1x10-14 [OH-]
- 21. pH pH + pOH = 14 pOH [H+] = [OH-]= 10-pH 10-pOH [H+] [H+][OH-]=1x10-14 [OH-]
- 22. Calculate pH ↔ pOH What is the pH of a solution with pOH = 13? Is the solution acidic or basic? What is the pOH of a solution with pH = 8? Is the solution acidic or basic? What are the concentrations of H+ and OH-?
- 23. Calculate pH ↔ pOH What is the pH of a solution with pOH = 13? Is the solution acidic or basic? pH= 1, acidic What is the pOH of a solution with pH = 8? Is the solution acidic or basic? pOH = 6, basic What are the concentrations of H+ and OH-? [H+] = 1.0x10-1, [OH-] = 1.0x10-13 [H+] = 1.0x10-8, [OH-] = 1.0x10-6