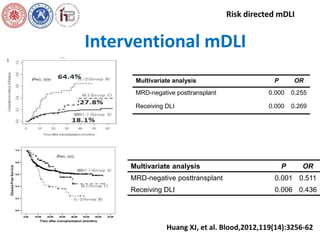
This document summarizes research on risk stratification, prevention, and management of leukemia relapse after hematopoietic stem cell transplantation (HSCT). It discusses:
1. Use of modified donor lymphocyte infusions to establish graft-versus-leukemia effects without severe graft-versus-host disease.
2. Research in t(8;21) acute myeloid leukemia showing allo-HSCT can improve outcomes for high-risk patients, and minimal residual disease monitoring post-HSCT better predicts risk than c-KIT mutations.
3. Studies demonstrating imatinib after allo-HSCT from related donors improves outcomes for Philadelphia chromosome-positive leukemias by preventing relapse when guided by