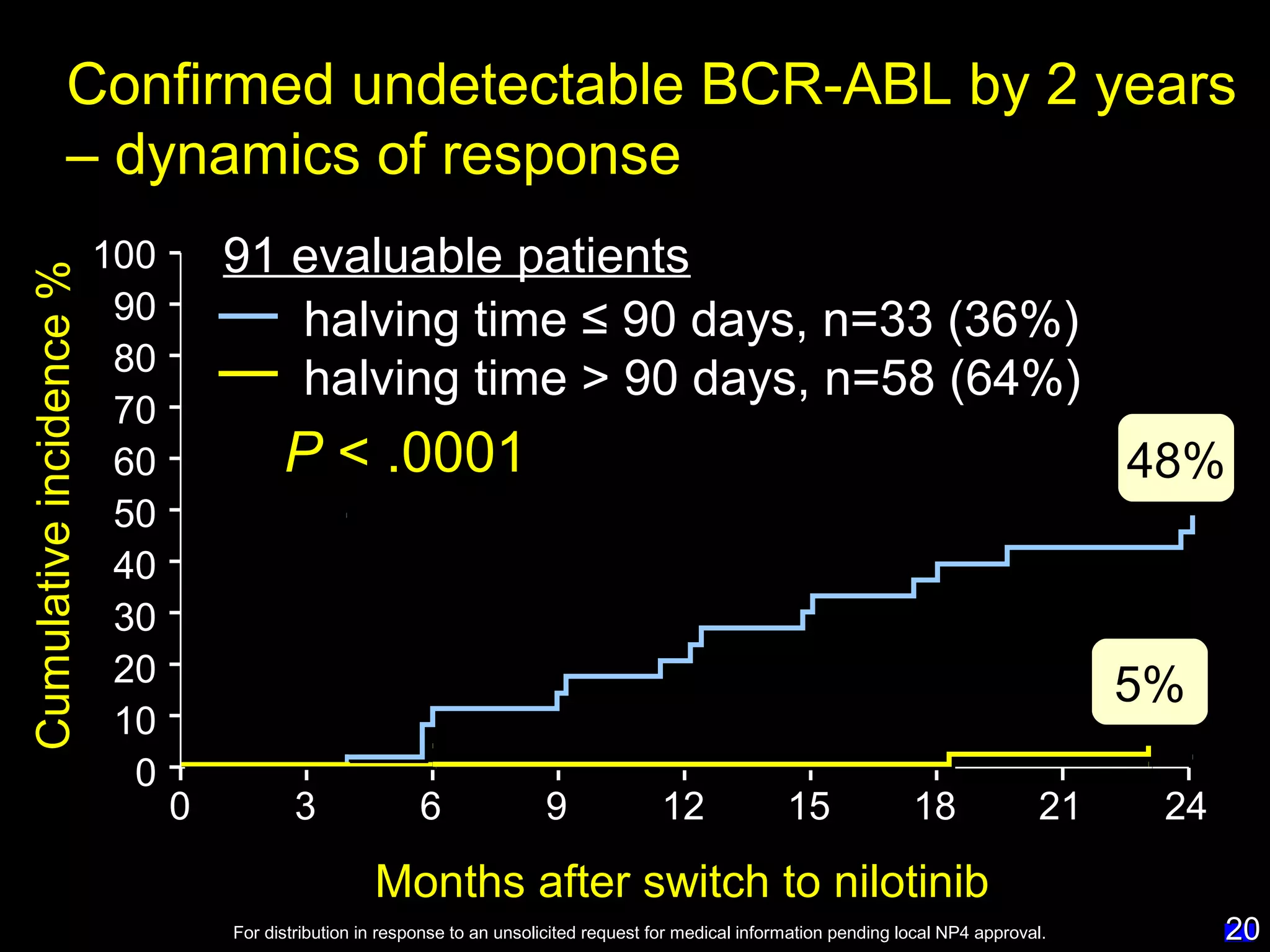
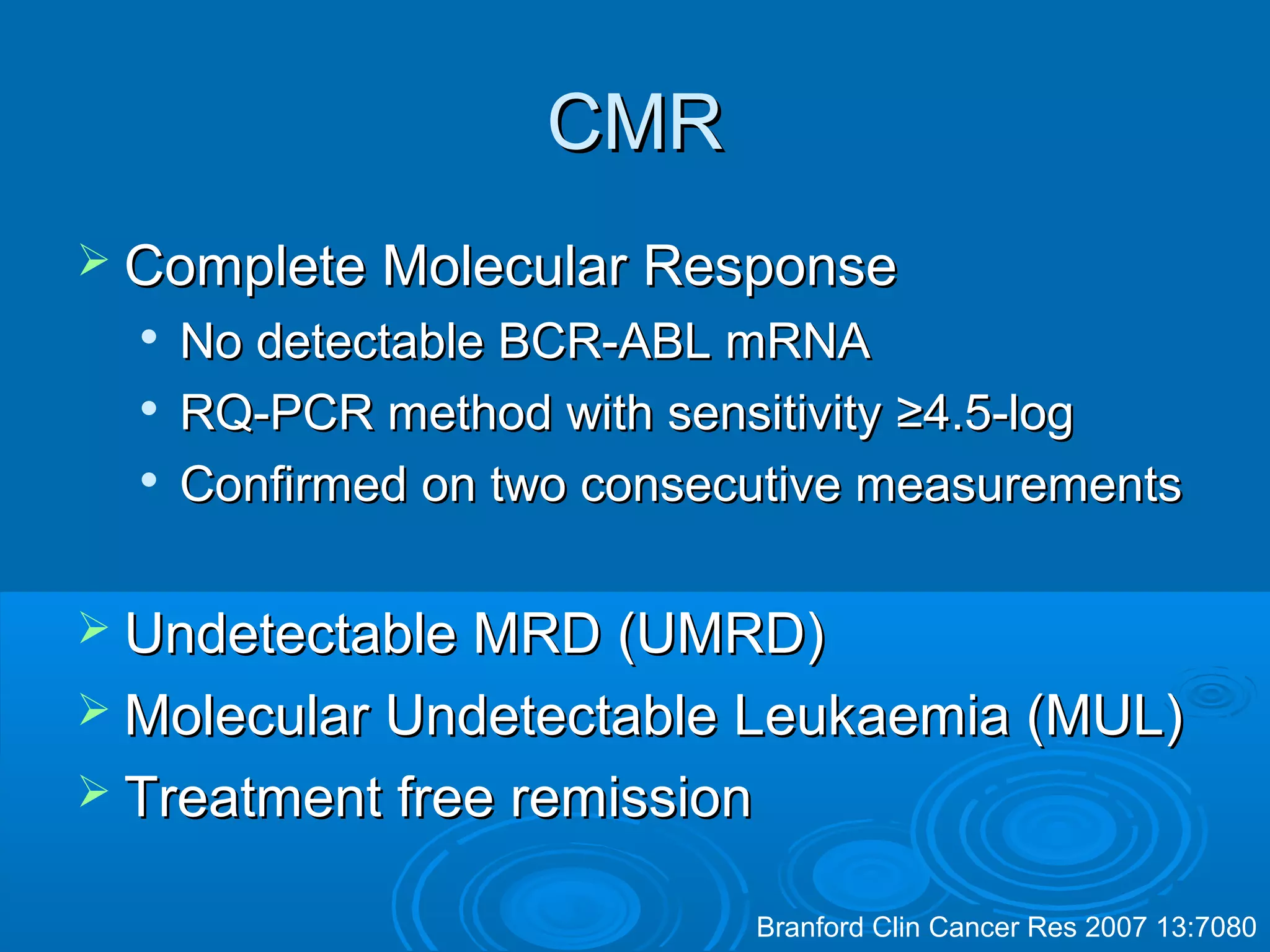
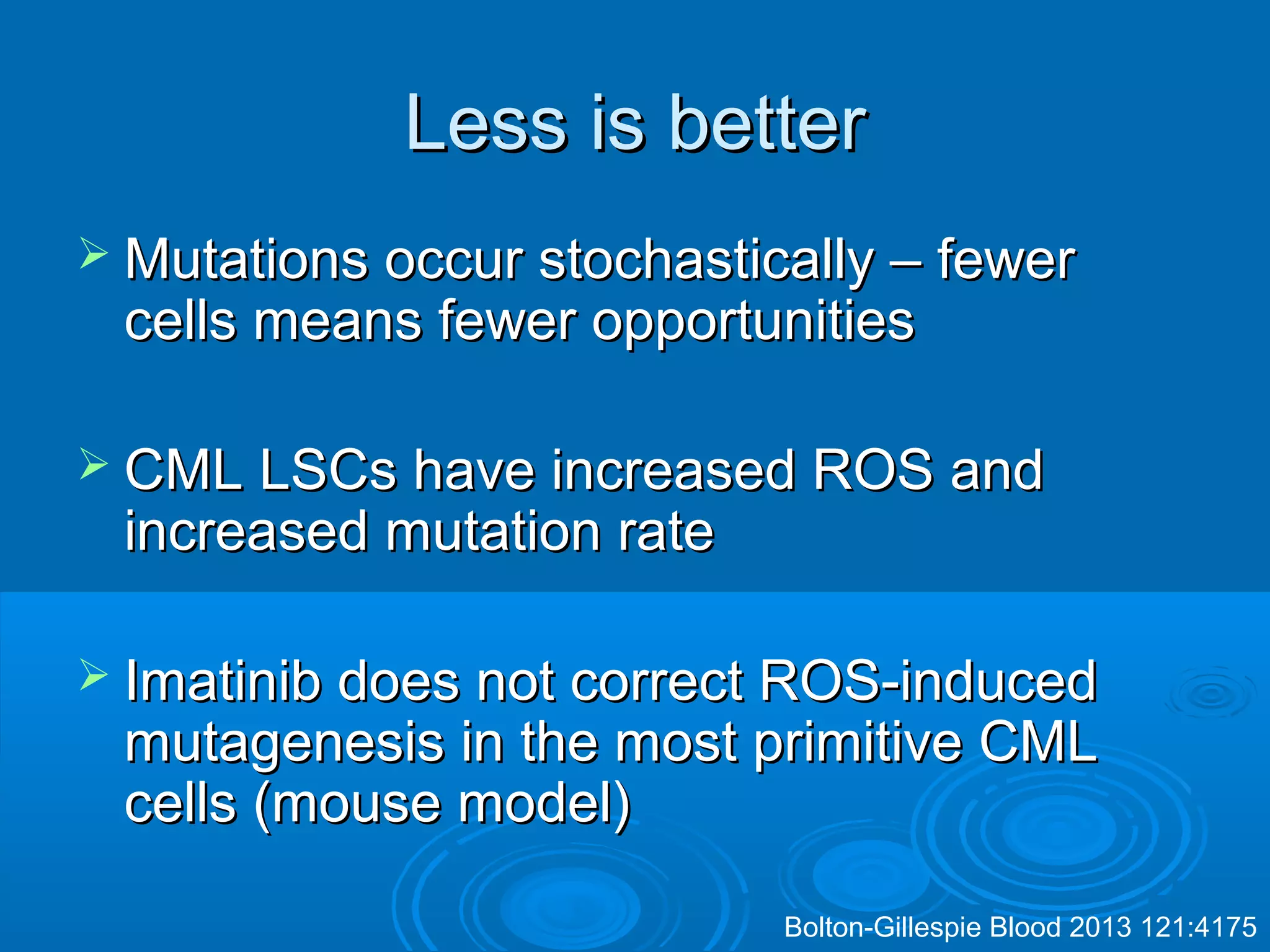
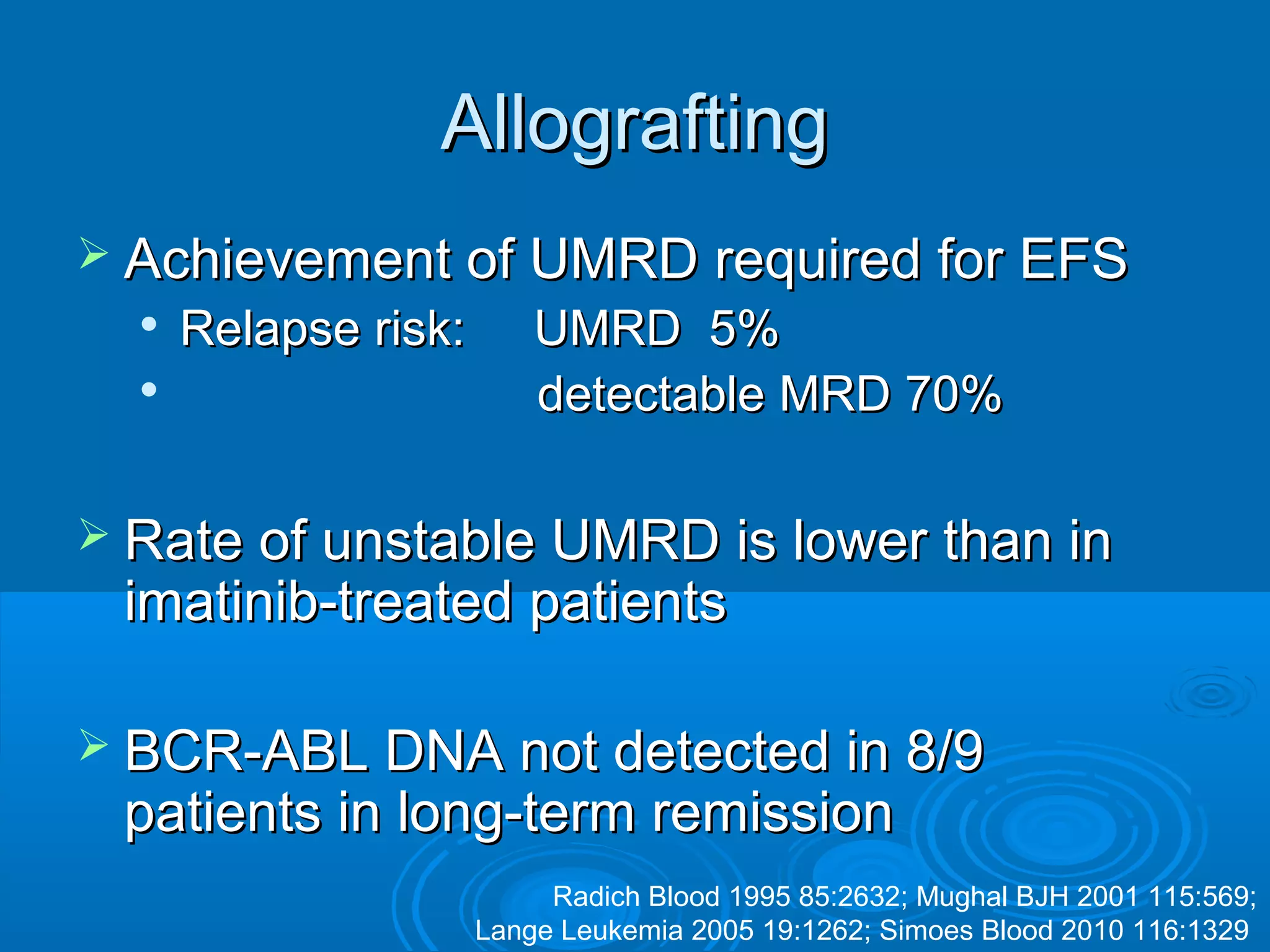
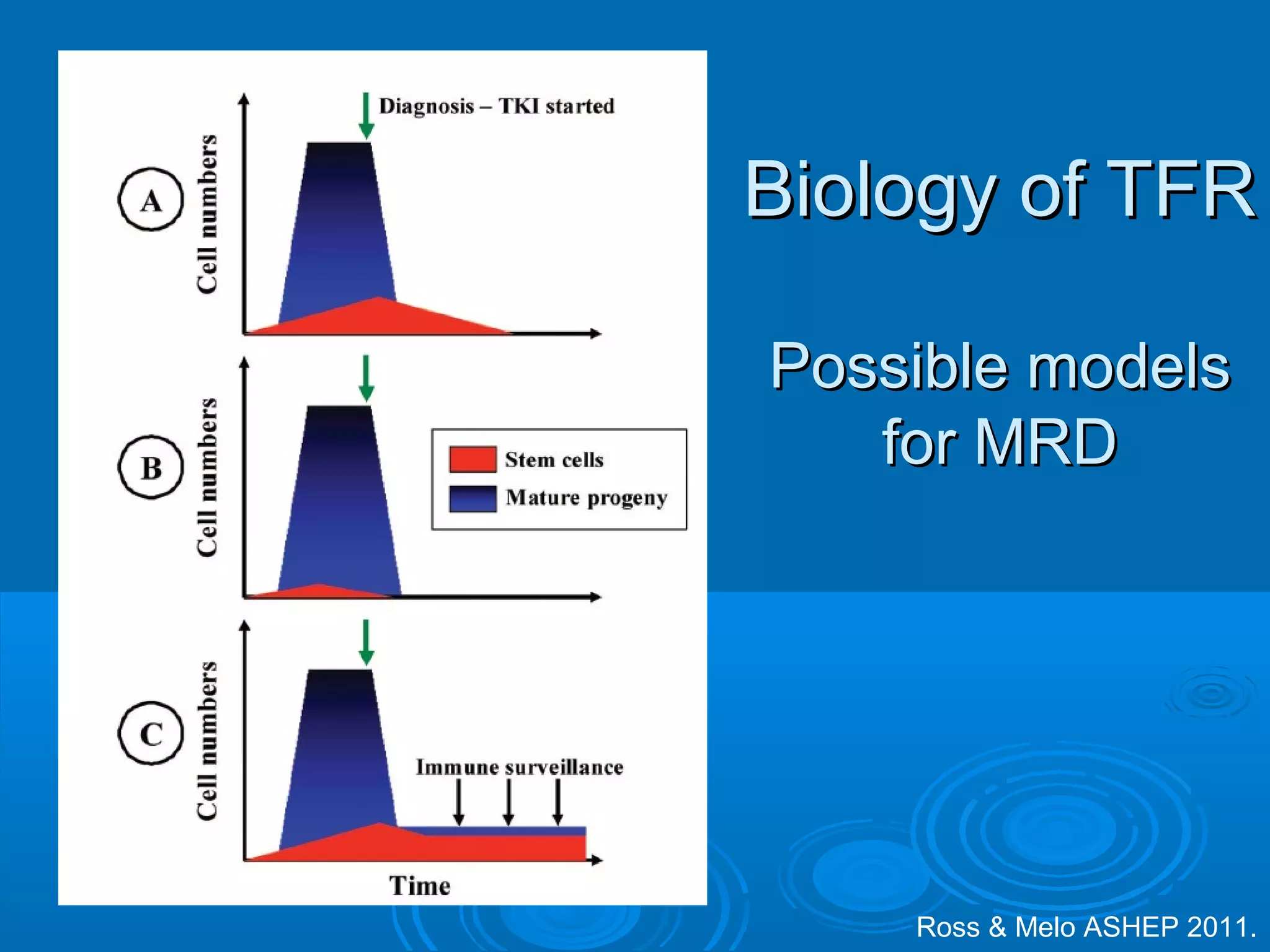
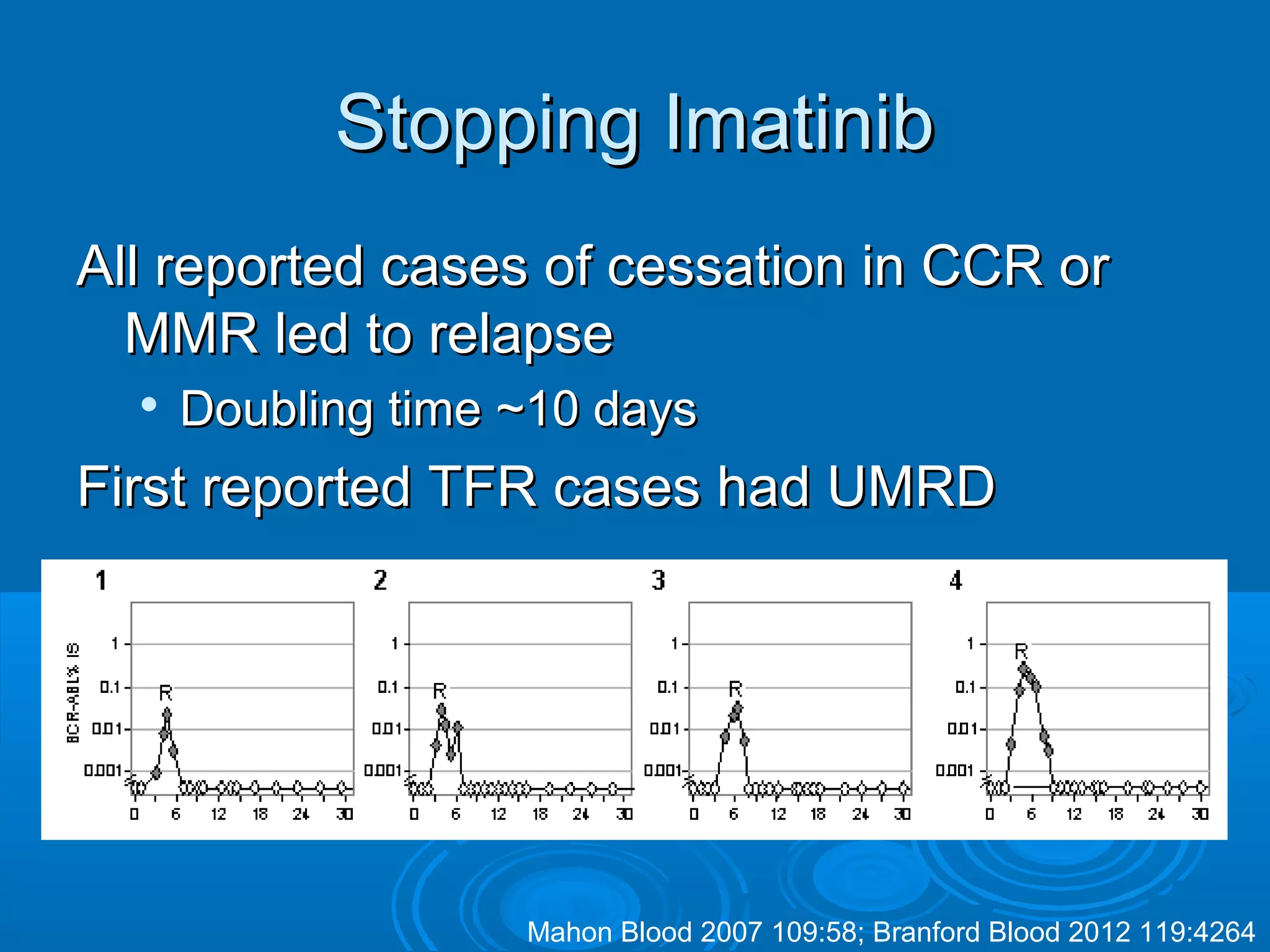
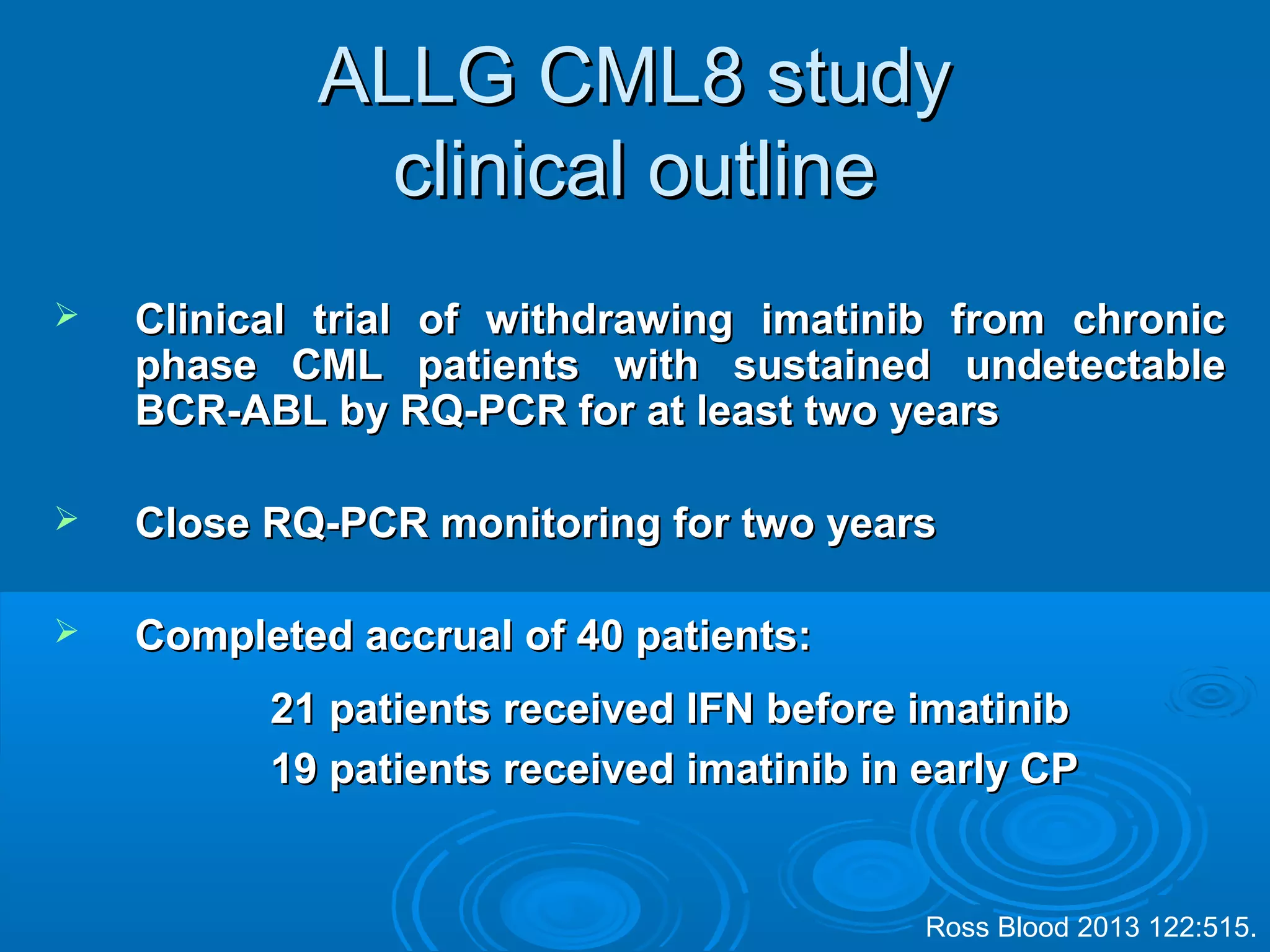
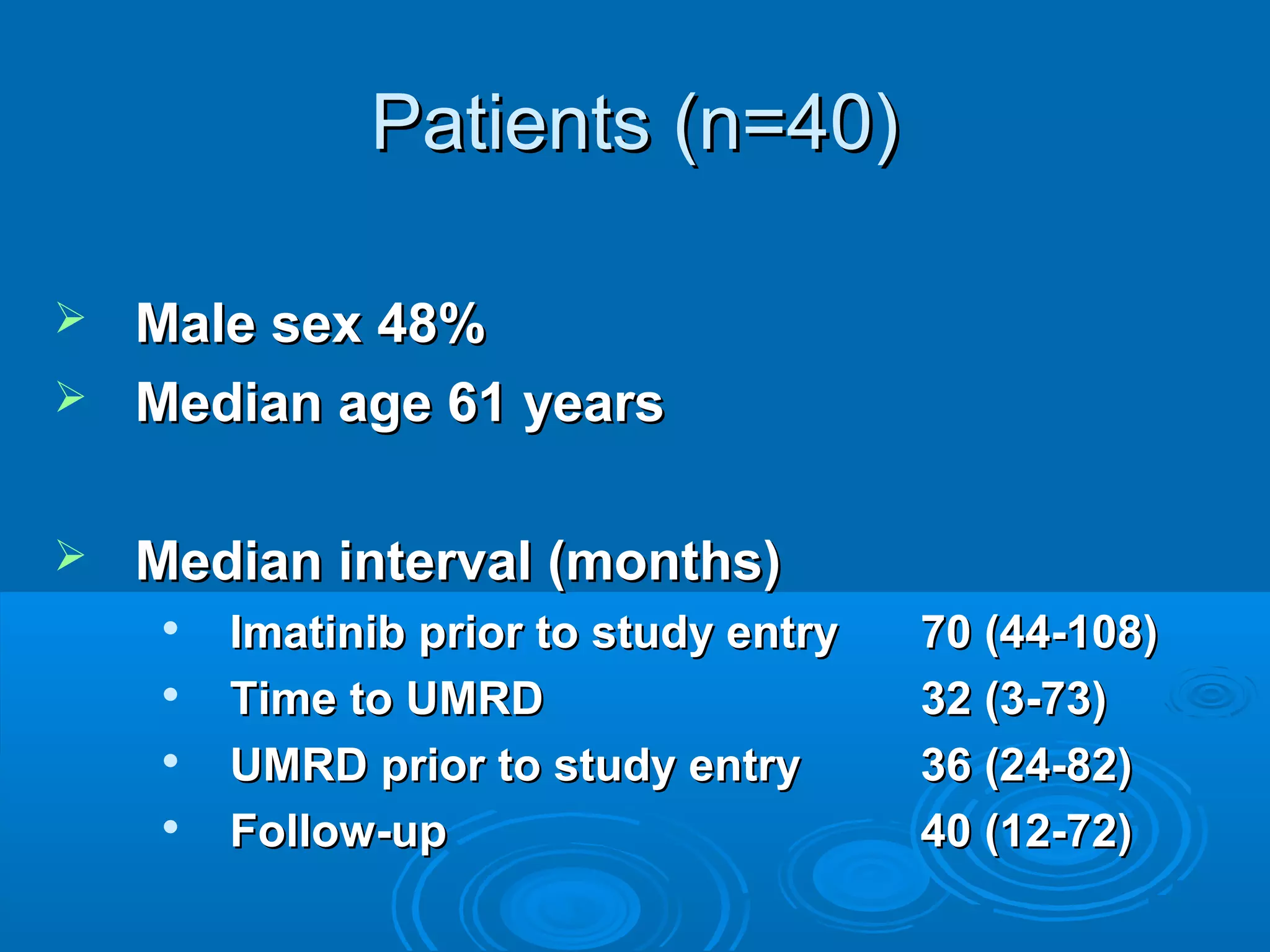
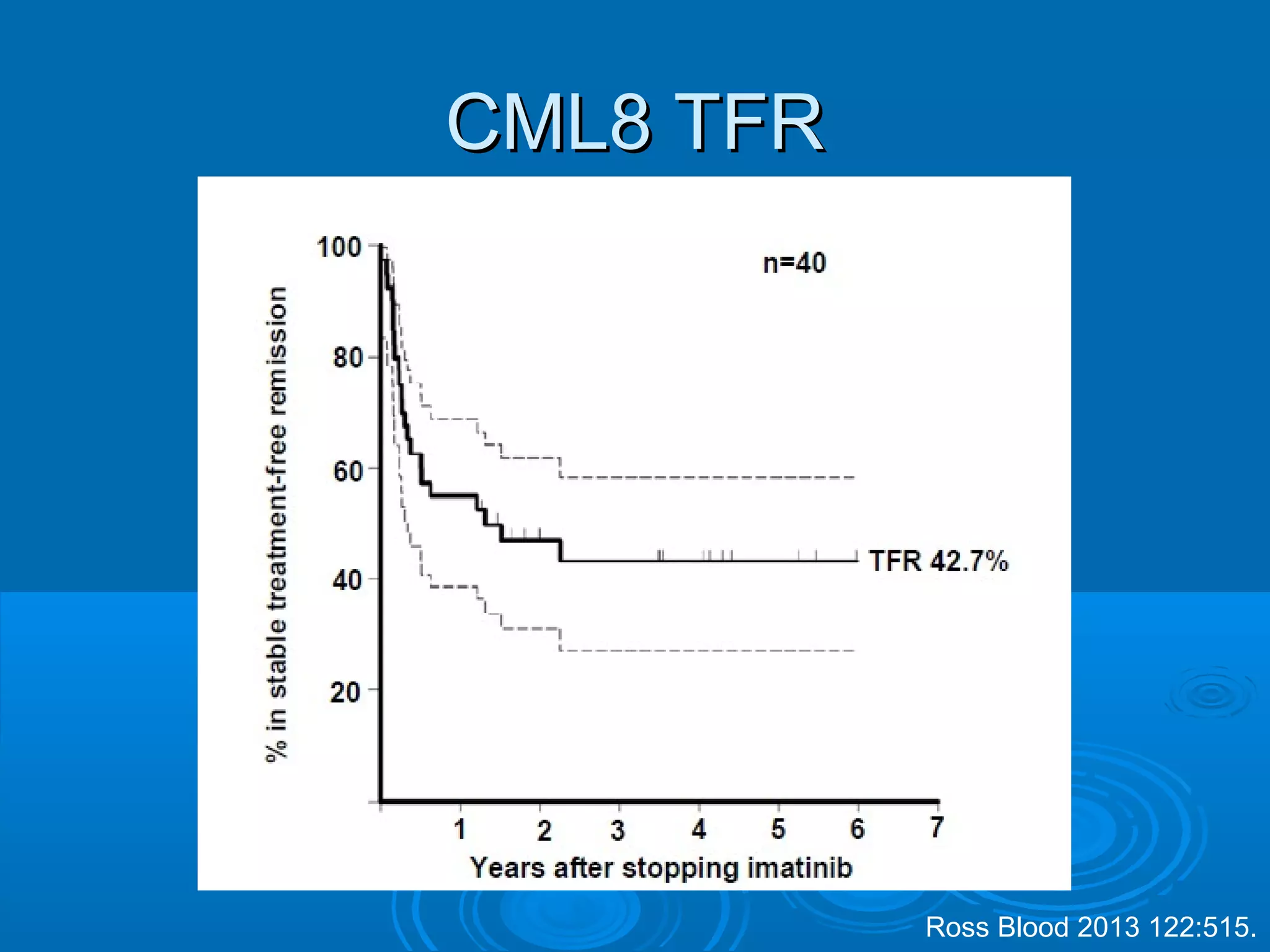
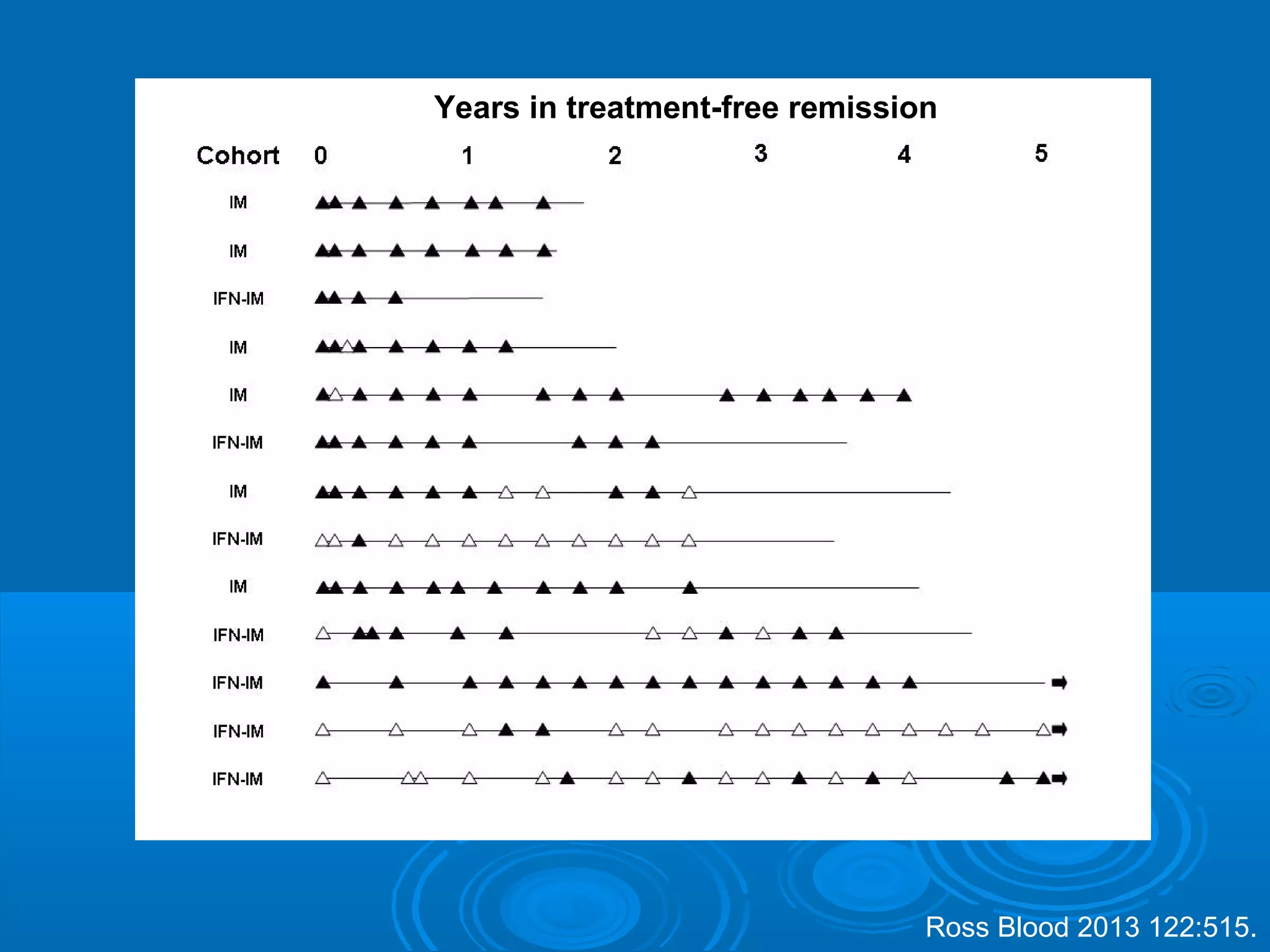
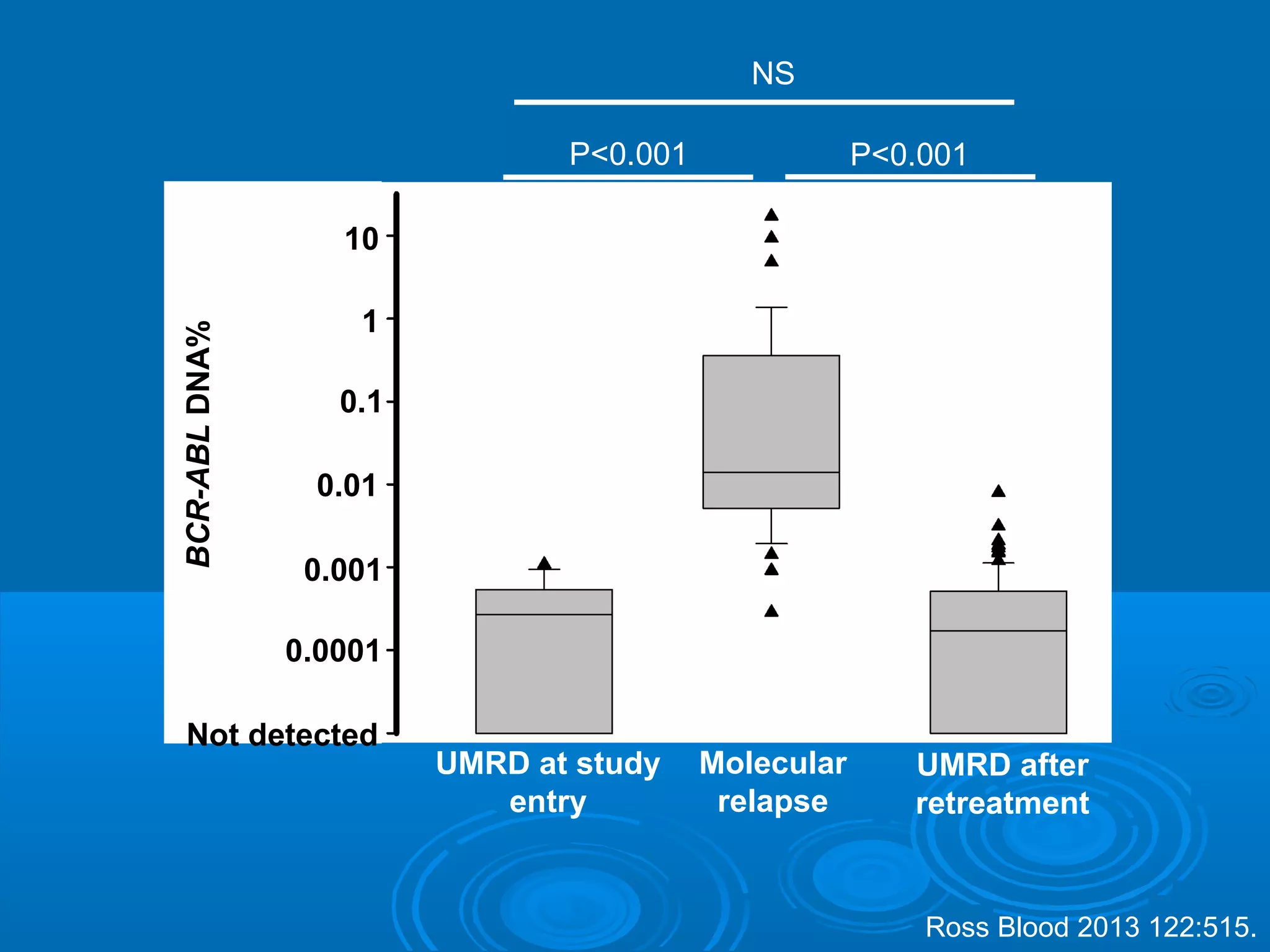
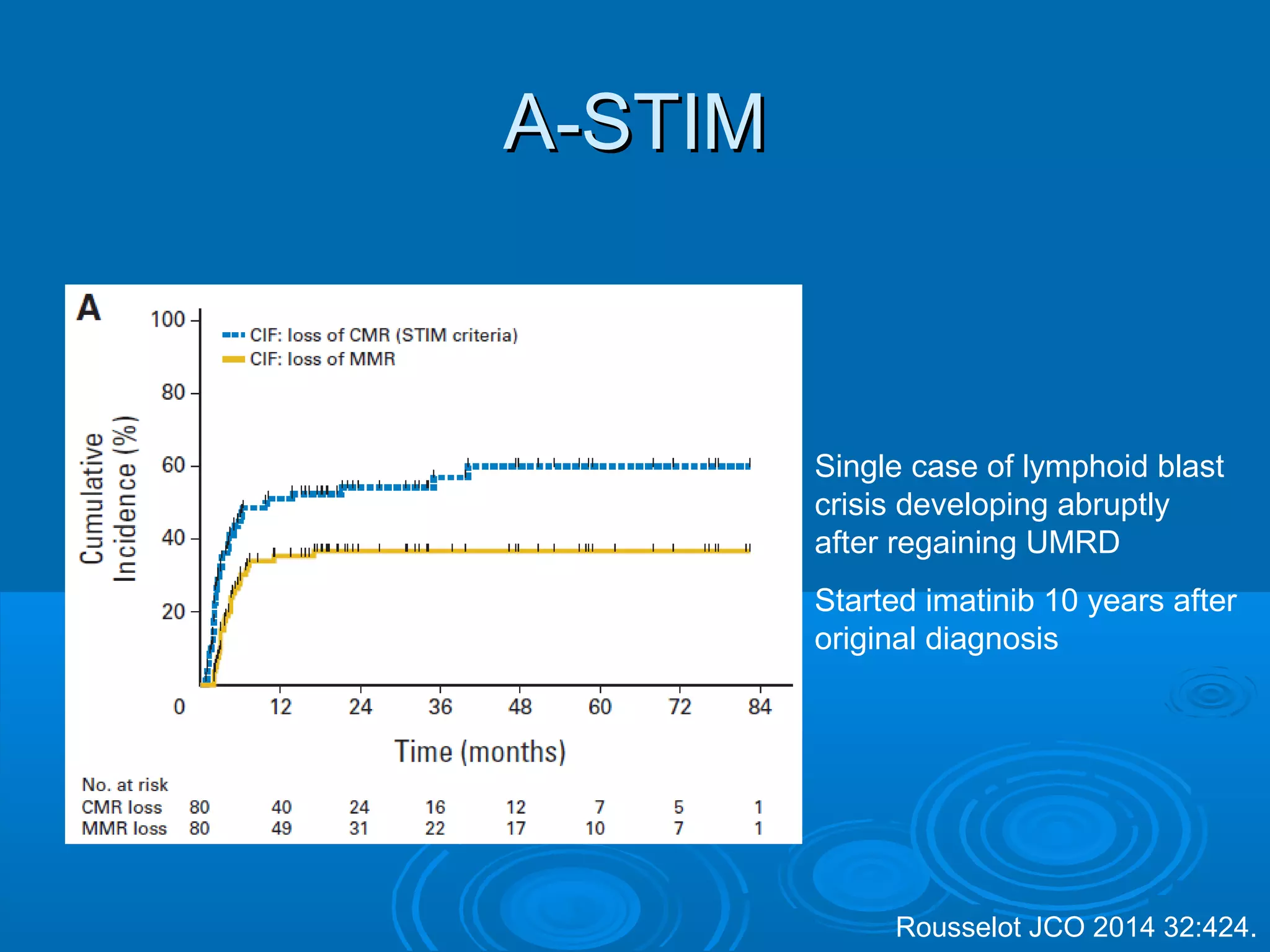
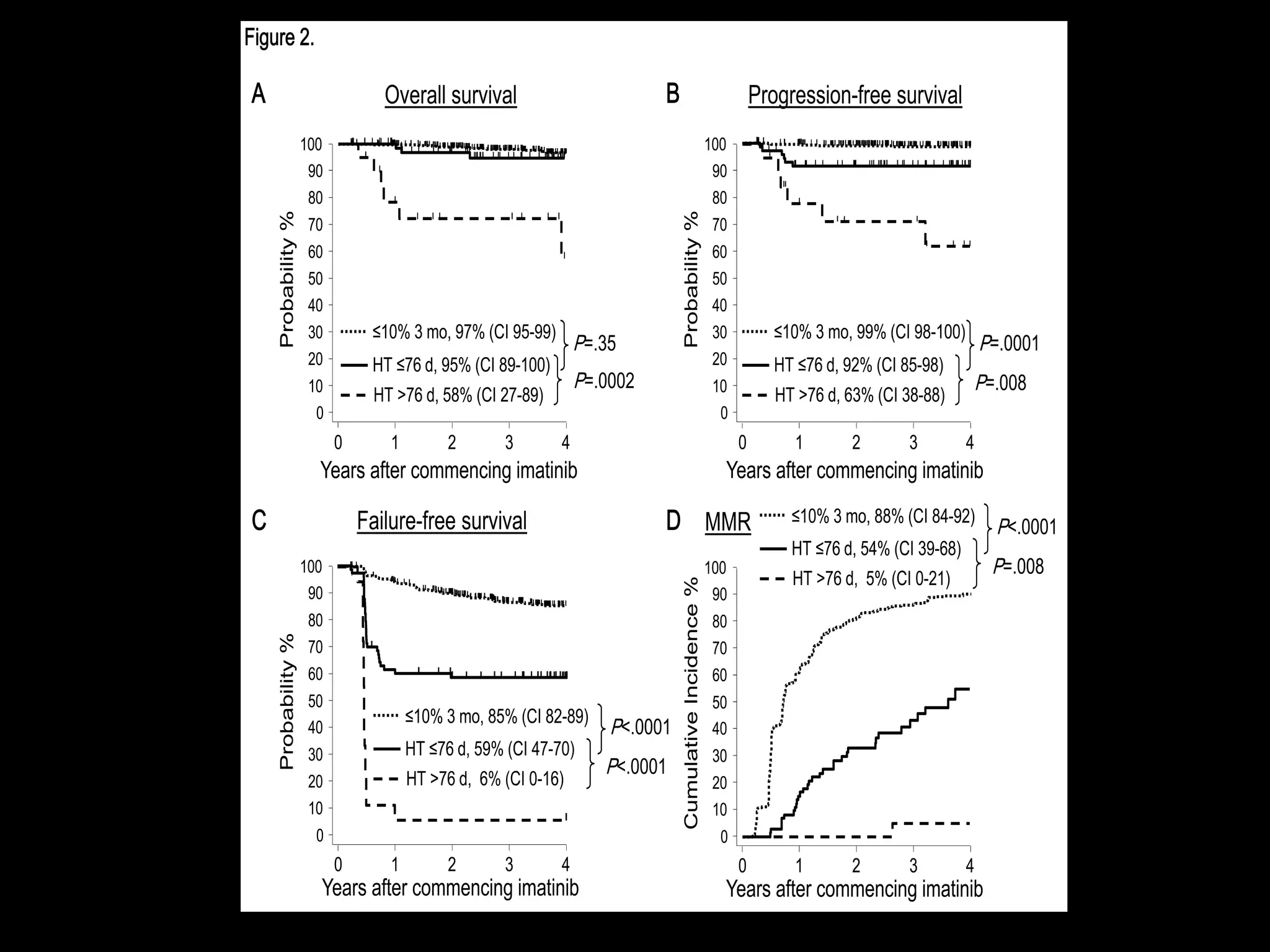
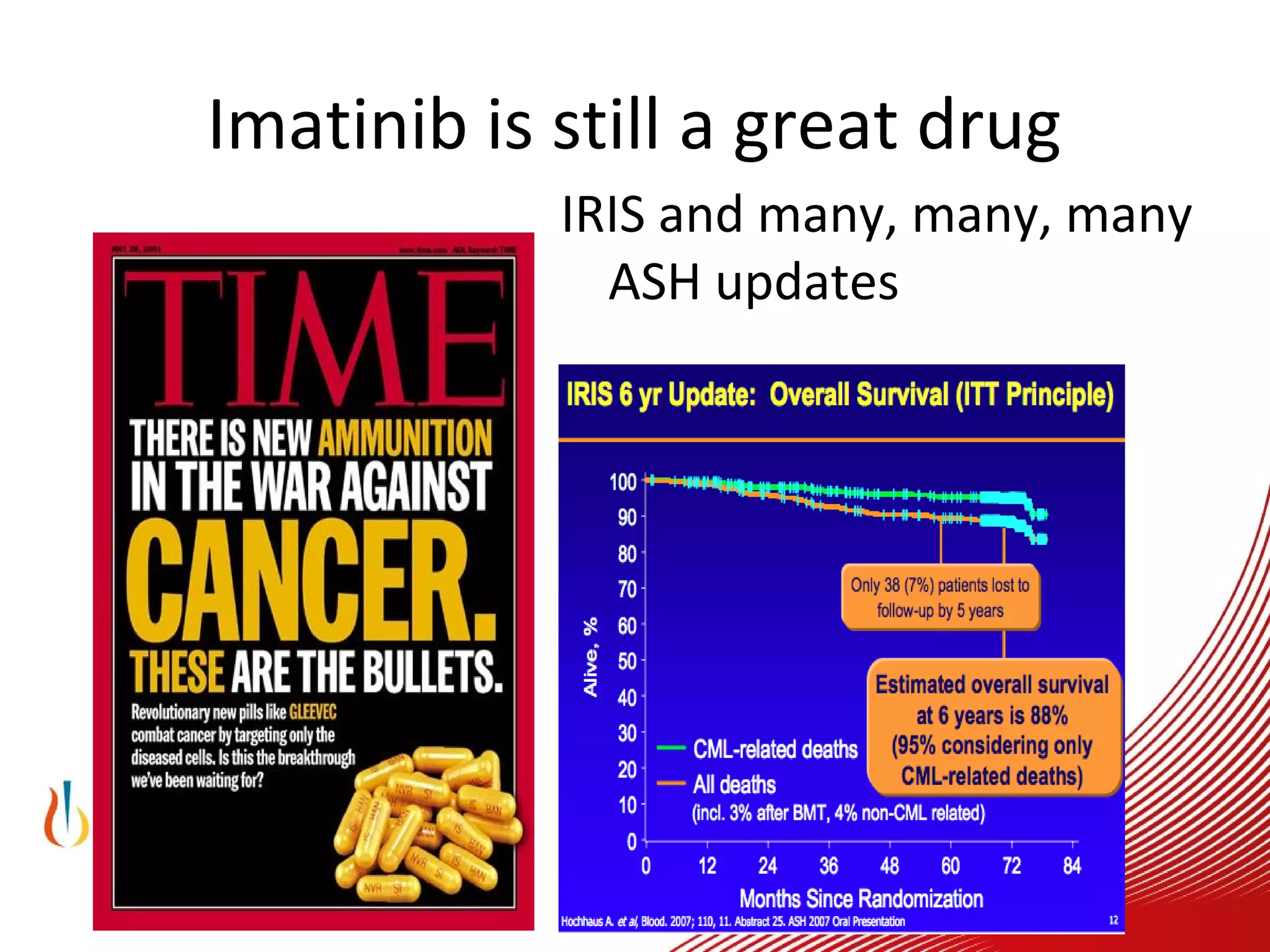
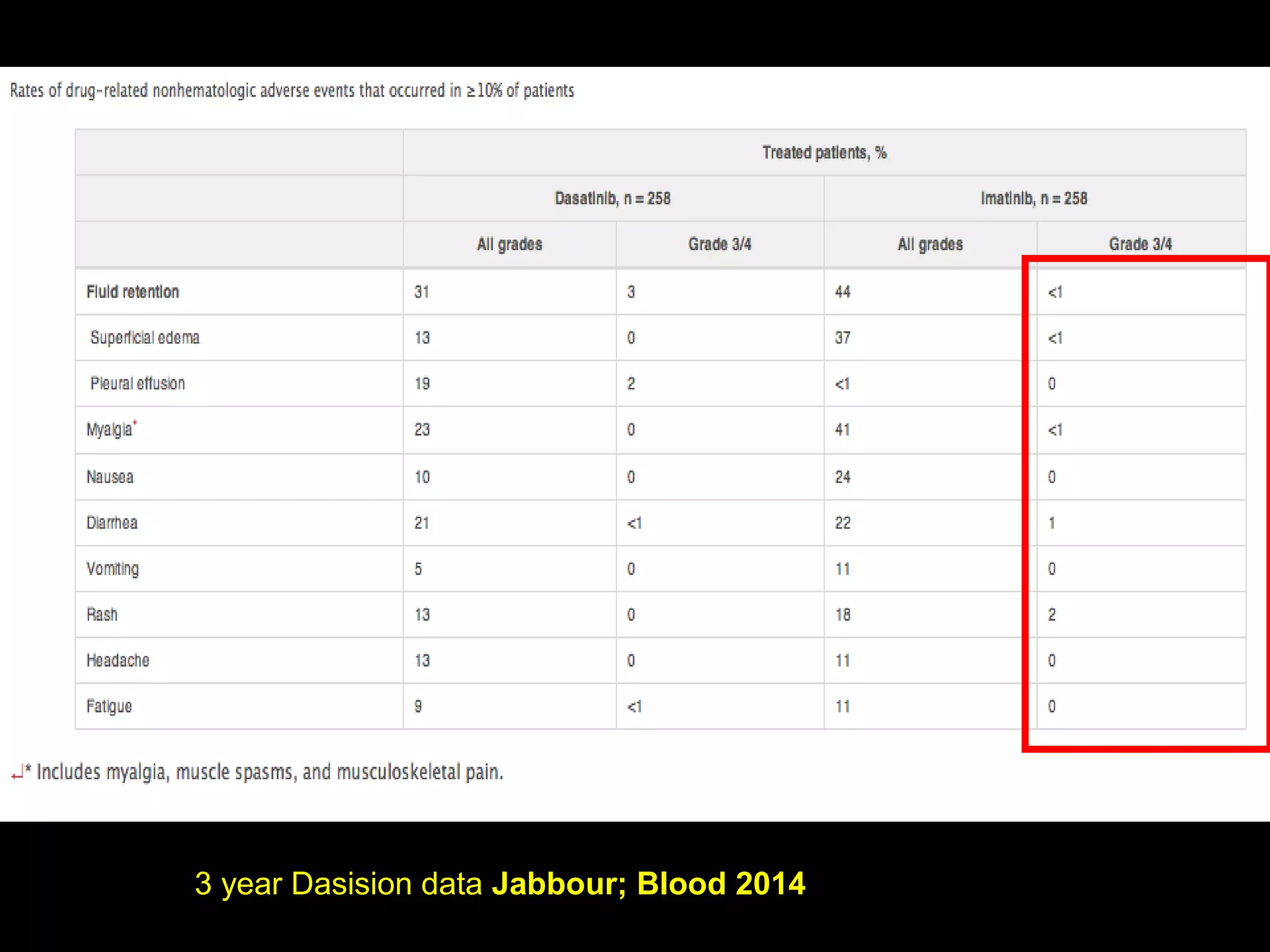
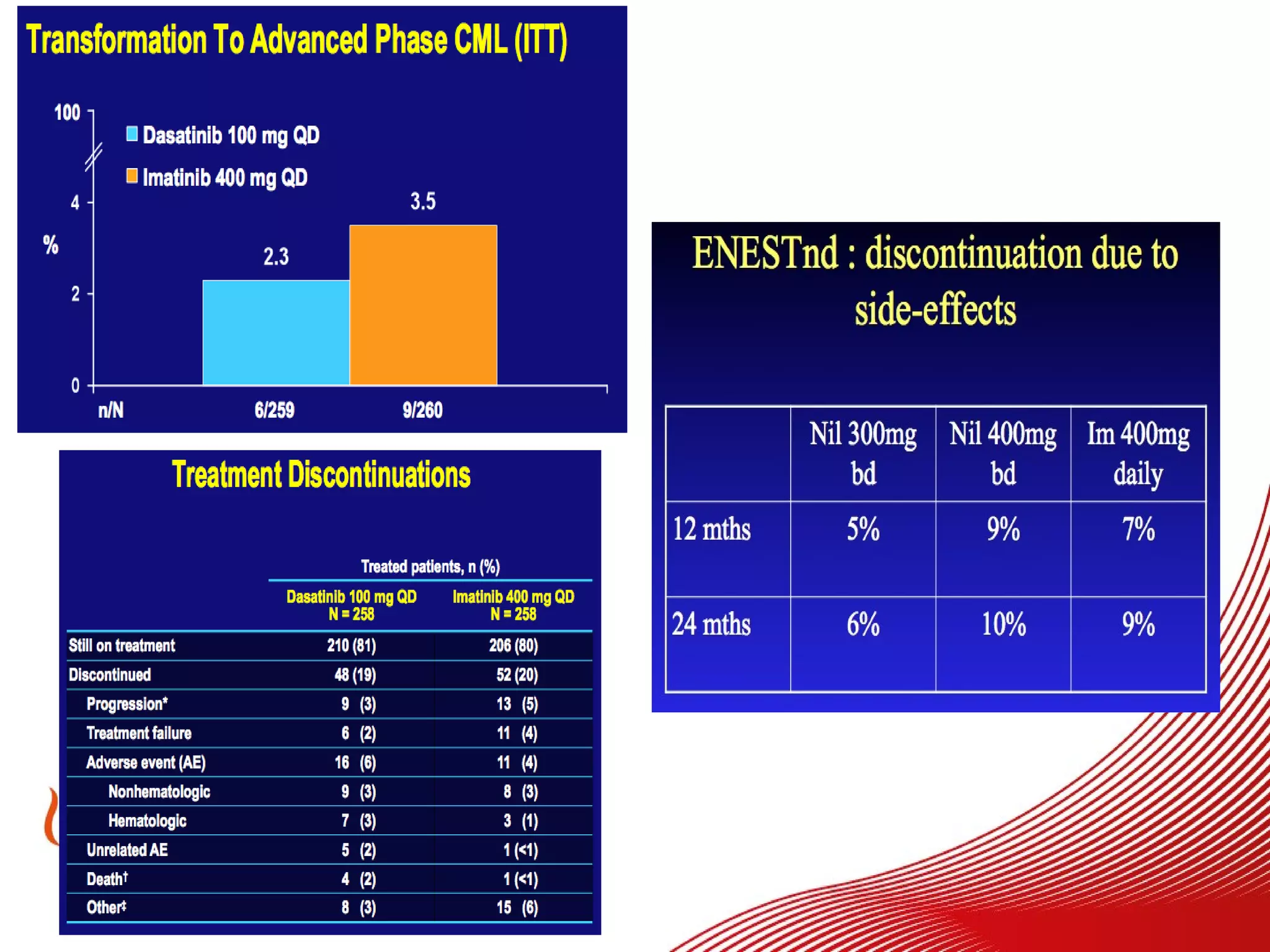
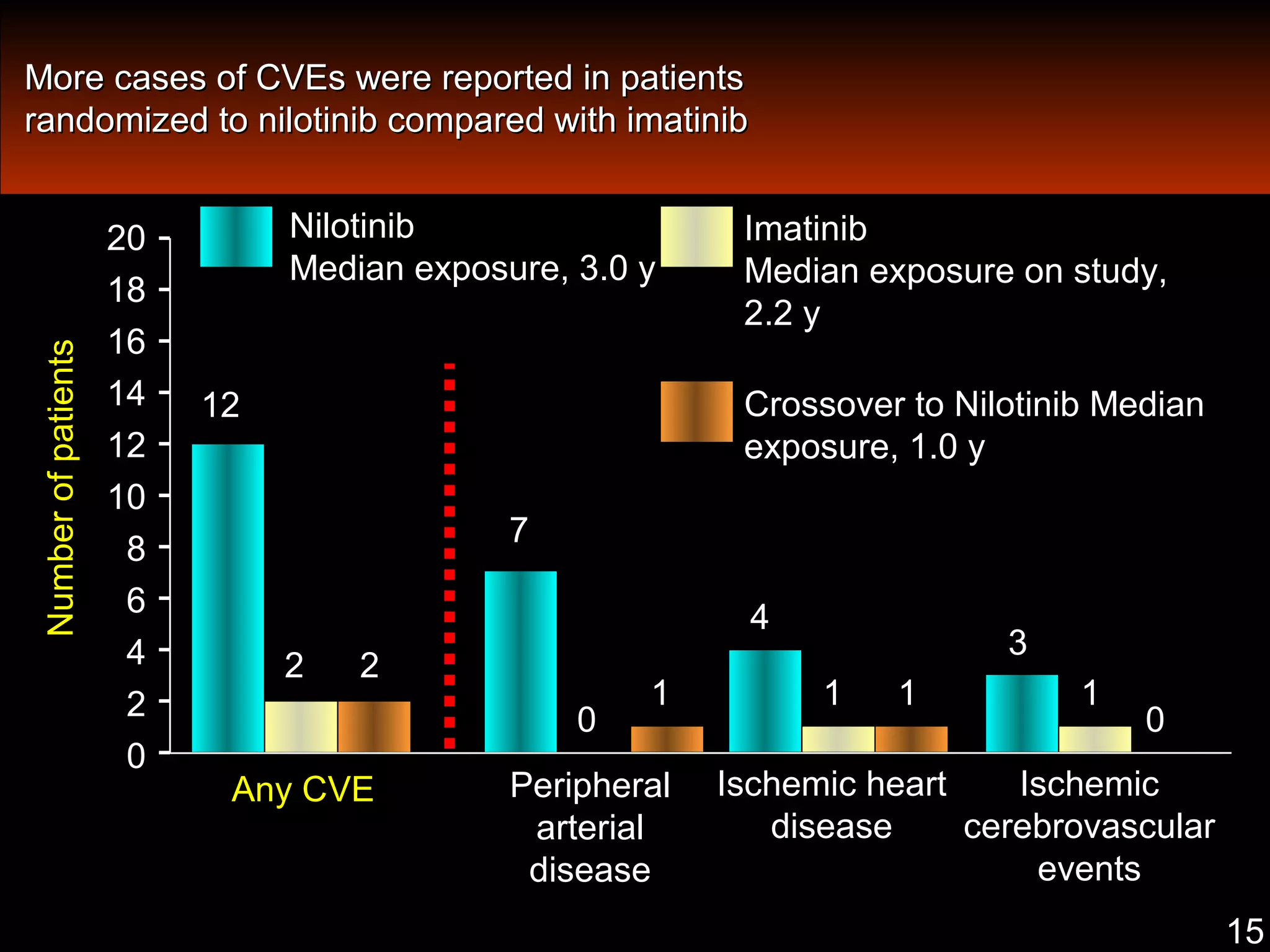
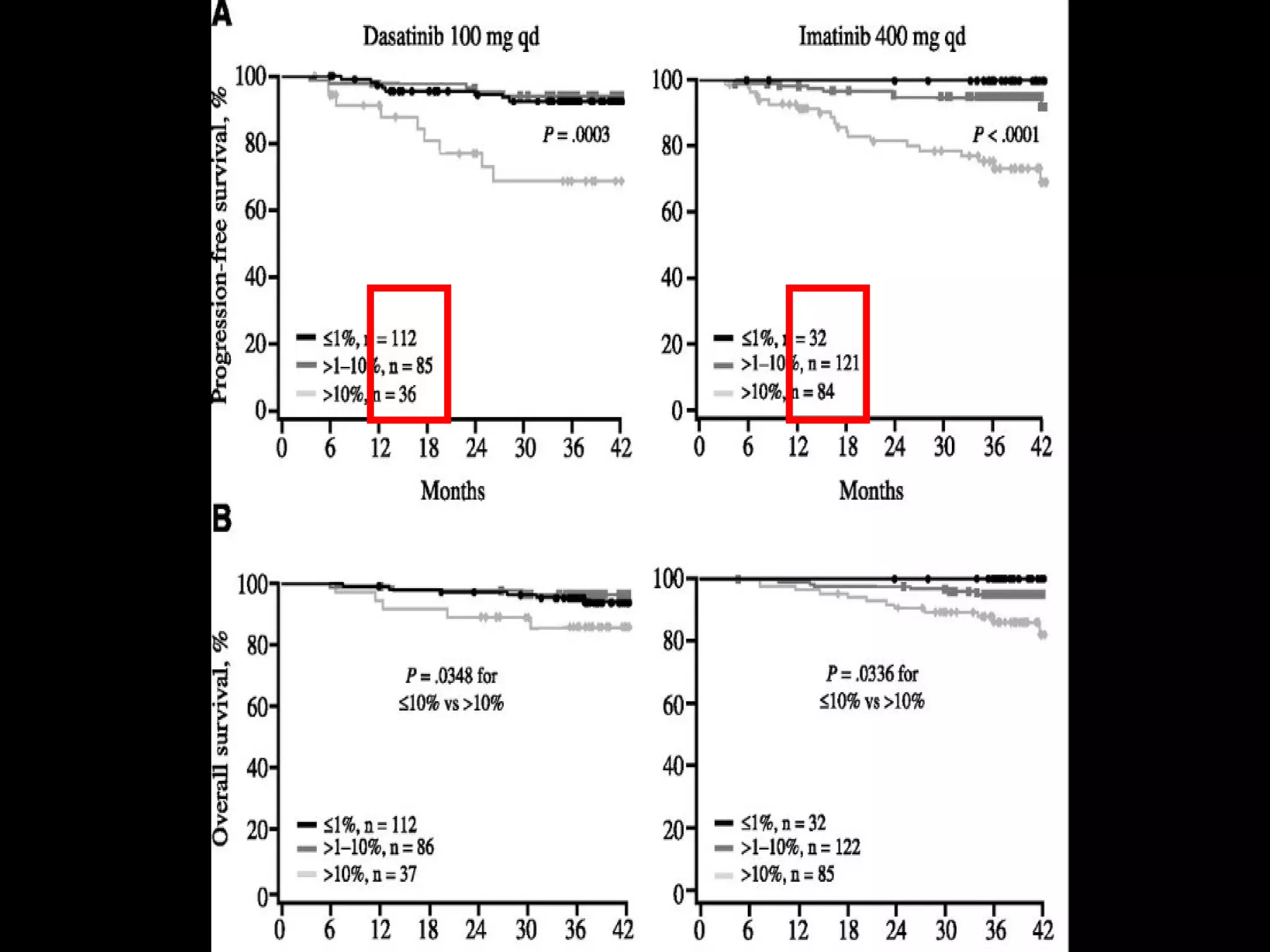
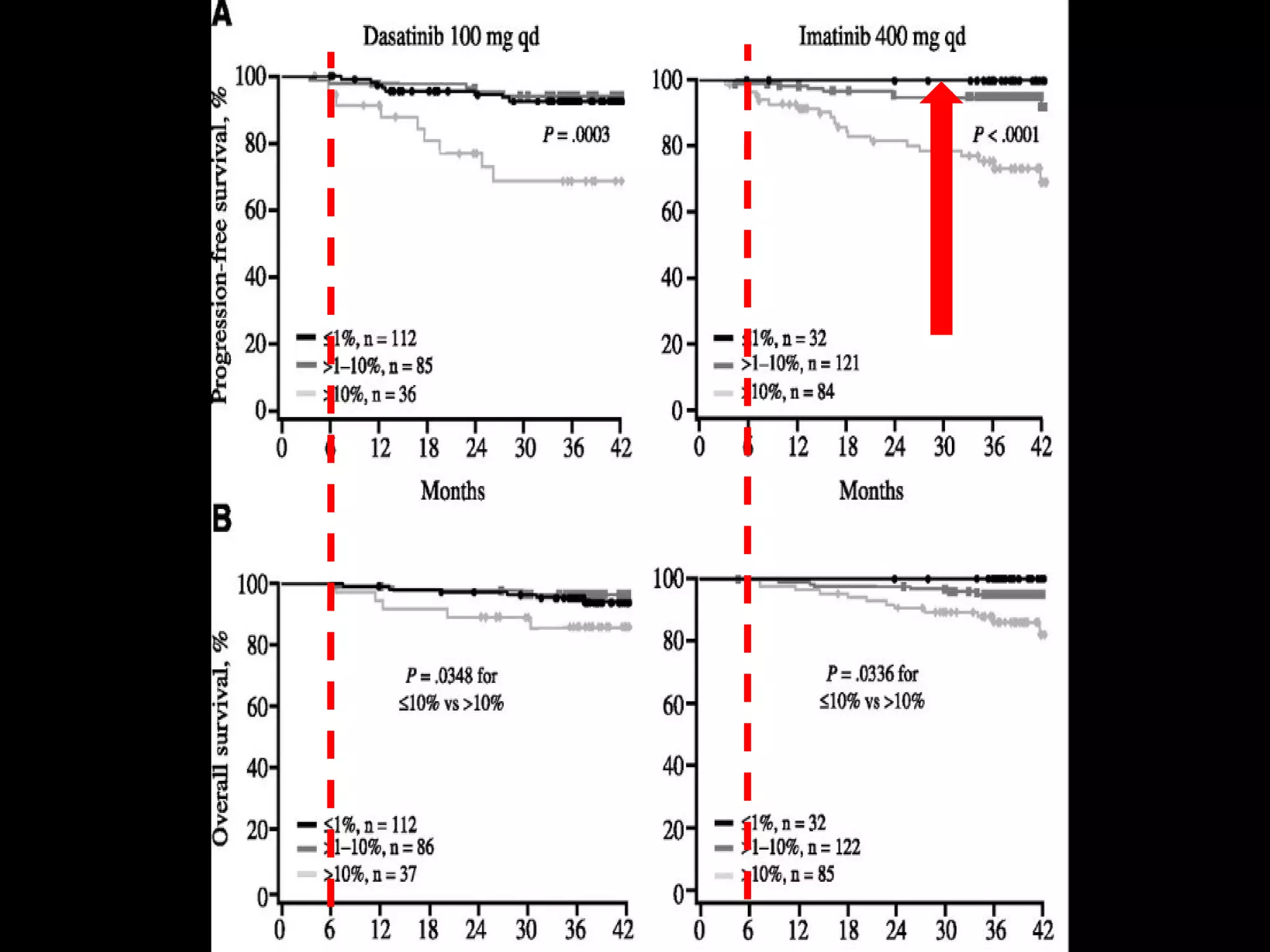
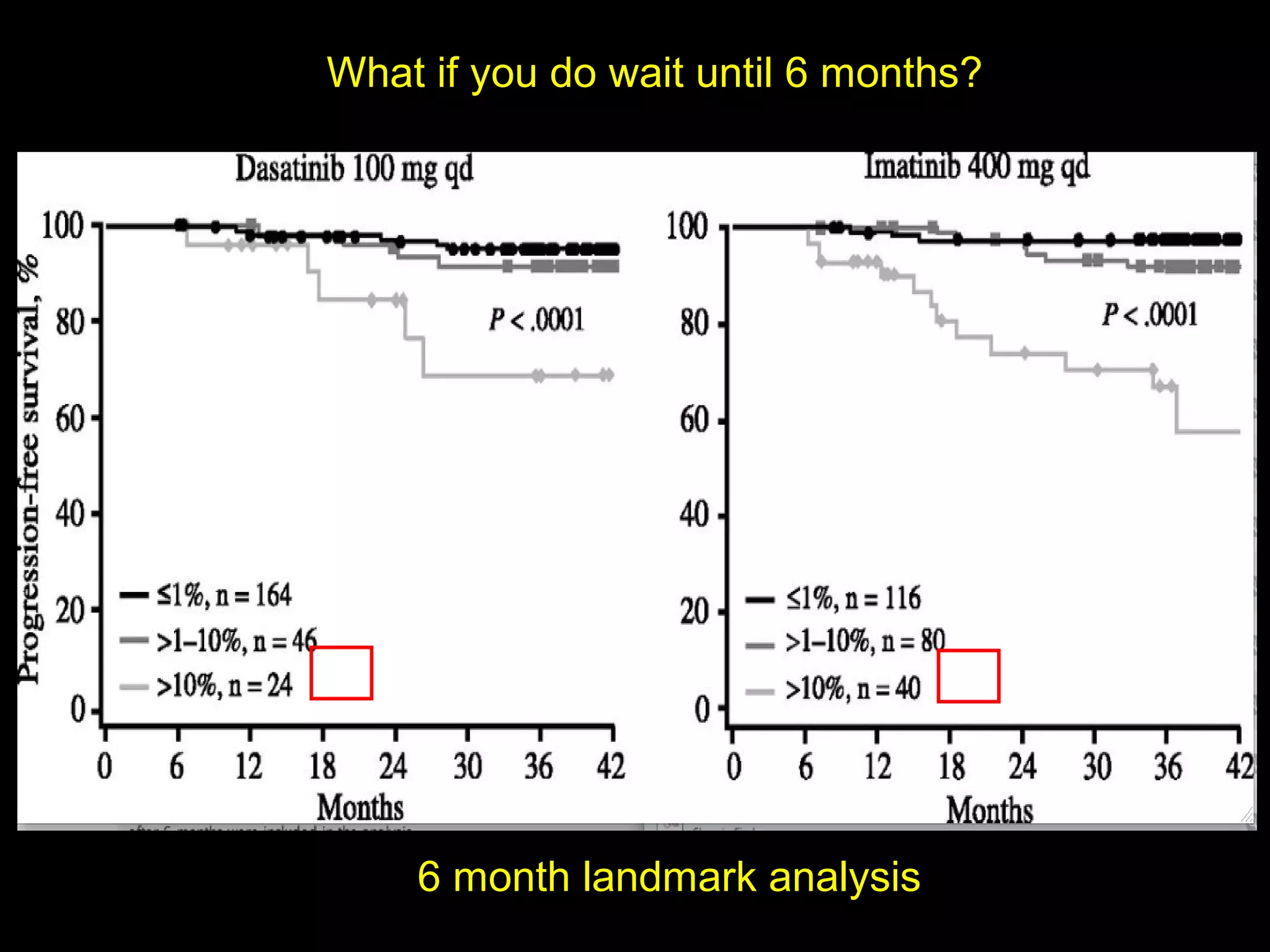
This document discusses when to stop tyrosine kinase inhibitor (TKI) treatment in chronic myeloid leukemia (CML) patients. There are three situations when TKI treatment may be stopped: 1) when the TKIs are no longer effective, 2) when the TKIs become too toxic, and 3) when a complete molecular response (CMR) has been achieved, indicating the TKIs appear to have cured the disease. Achieving an undetectable minimal residual disease (UMRD) state, with no detectable BCR-ABL mRNA, is associated with a low relapse risk. Some CML patients are able to stop TKI treatment long-term after achieving a UMRD, but stopping treatment















![OPTIMAL WARNING FAILURE
BASELINE NA -HIGH RISK,
-CCA/Ph+
(Major route)
NA
3 mo Ph+ ≤ 35% and/or
BCR-ABL ≤ 10%
Ph + 36-95% and/or
BCR-ABL ≥ 10%
No CHR and/or
Ph + > 95%
6 mo Ph+ 0 and/or
BCR-ABL < 1%
Ph + 1-35% and/or
BCR-ABL 1-10%
Ph + > 35% and/or
BCR-ABL > 10%
12 mo BCR-ABL≤ 0.1% BCR-ABL 0.1-1 % Ph + ≥ 1%, and/or
BCR-ABL > 1%
24 mo BCR-ABL ≤ 0.1% BCR-ABL 0.1-1% BCR-ABL > 1%
EUROPEAN LEUKEMIANET 2013
RESPONSE TO TREATMENT FIRSTLINE
Baccarani M et al, Blood. 2013 Jun 26. [Epub ahead of print].Yellow = cytogenetic response](https://image.slidesharecdn.com/8ritchiecmlbtg2015-150518165038-lva1-app6891/75/When-to-stop-TKI-in-Chronic-Myelogenous-Leukemia-40-2048.jpg)