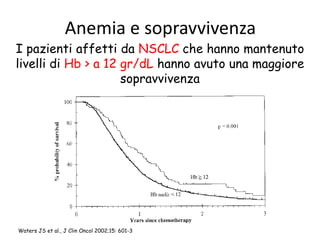
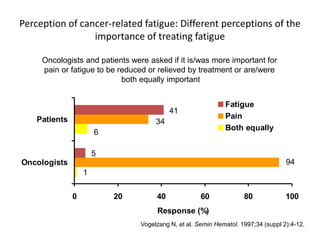
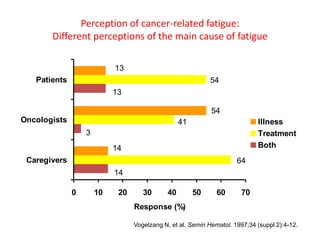
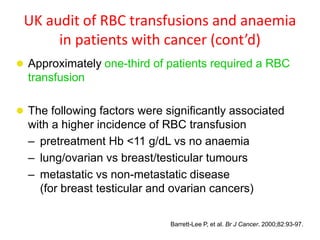
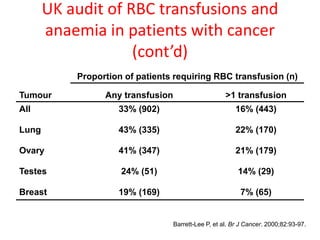
This document discusses anaemia in cancer patients. Some key points:
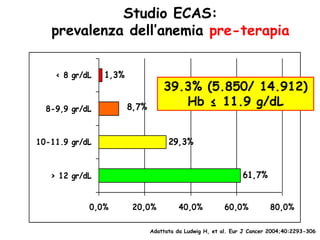
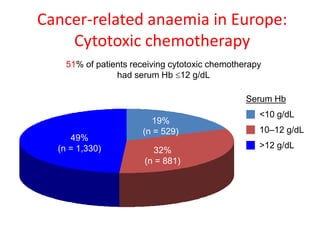
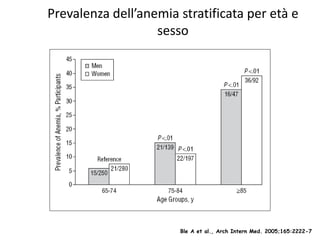
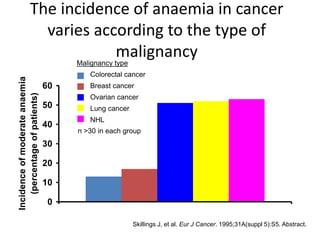
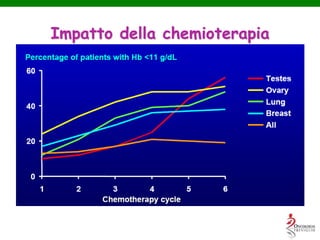
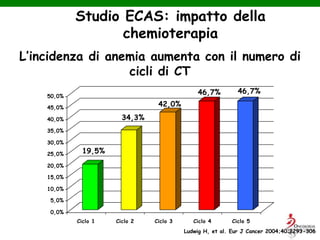
- Anaemia is very common in cancer patients, occurring in 20-60% at presentation and increasing with chemotherapy cycles.
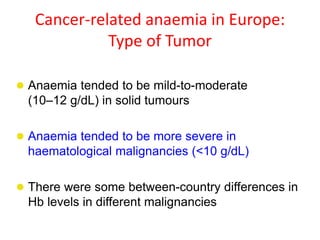
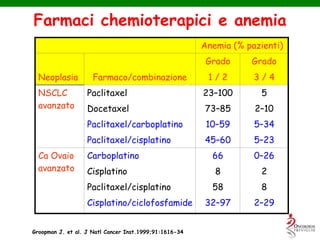
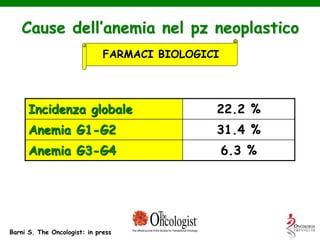
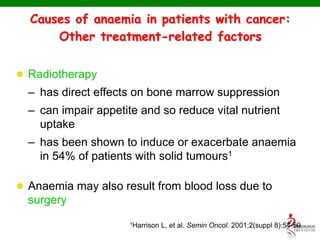
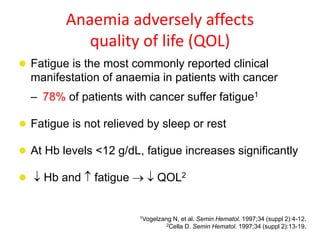
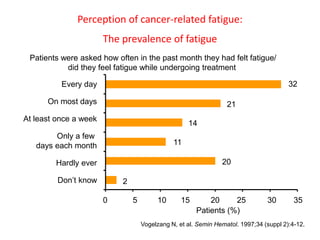
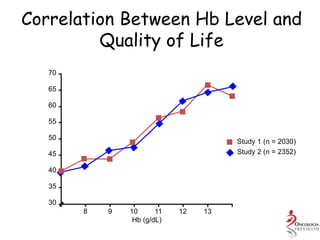
- Anaemia can be caused by the cancer itself, chemotherapy, radiotherapy, blood loss from surgery. It adversely impacts quality of life through fatigue and other symptoms.
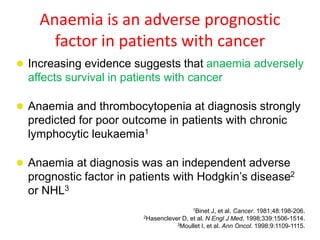
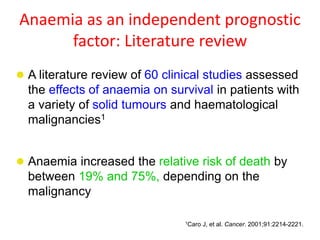
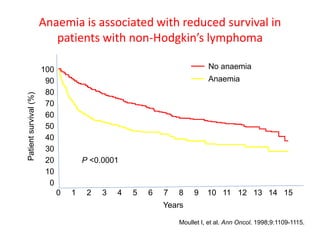
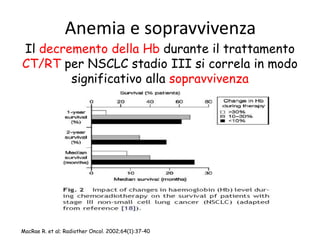
- More severe anaemia is associated with worse survival outcomes and prognosis. This may be due to tumour hypoxia enhancing progression.
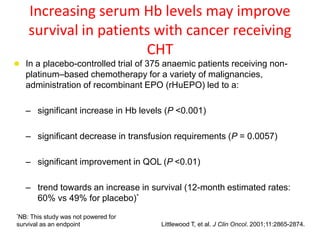
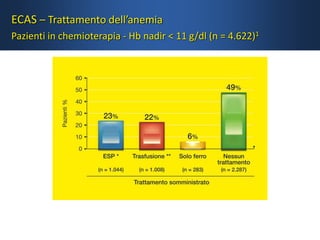
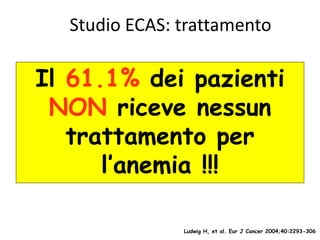
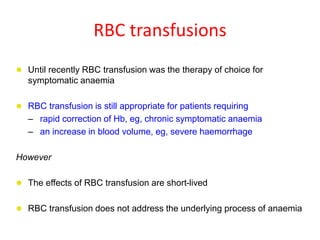
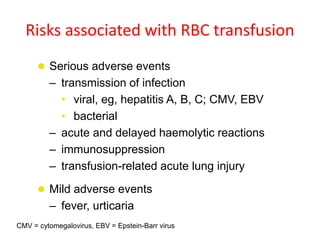
- While red blood cell transfusions are used to treat anaemia, over a third of anaemic cancer patients receive no treatment. Recombinant erythropoietin can increase hemoglobin levels and





![53 studi clinici randomizzati
N=13.933
Mortalità in studio
HR = 1.17 [95% CI, 1.06-1.30] p=0.003
Overall Survival
HR = 1.06 [95% CI, 1.00-1.12] p=0.0046
Nei pazienti anemici il trattamento con ESA
aumenta in modo statisticamente
significativo la mortalità e diminuisce la
sopravvivenza
Bohlius, Lancet 2009
Tutti i pazienti trattati con ESA
(popolazione globale anche fuori indicazione)](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-39-320.jpg)



![38 studi clinici randomizzati
N=10.441
Mortalità in studio
HR = 1.10 [95% CI, 0.98-1.24] p=0.12
Overall Survival
HR = 1.04 [95% CI, 0.97-1.11] p=0.263
Nei pazienti anemici in CHT non esiste
differenza statisticamente significativa tra
il gruppo dei pt trattati e quello controllo
in termini di mortalità e sopravvivenza
Bohlius, Lancet 2009
Solo pt con anemia indotta da CHT](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-45-320.jpg)
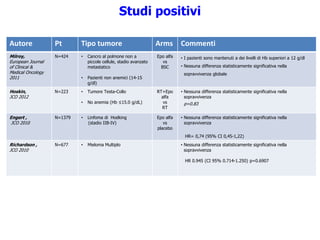
![Autore Pt Tipo tumore Arms Commenti
Pronzato,
The Oncologist 2010
N=223 • Cancro alla mammella
(~50% in stadio avanzato)
• Anemia lieve (Hb ≤12.0
g/dL)
Epo alfa
vs
BSC
• Nessuna differenza statisticamente significativa nella
sopravvivenza
HR= 1,05 (CI 95% 0,58-1,92) p=0.86
Aapro,
JCO 2008
N=463 • Cancro alla mammella
metastatico
• Hb< 12,9 g/dl
Epo beta
vs
control
• Nessuna differenza statisticamente significativa:
- Sopravvivenza globale
HR=1.07 (95% CI, 0.87 -1.33) p =0.522
- Sopravvivenza libera da progressione malattia
HR=1.07 (95% CI, 0.89-1.30) p=0.488
Cantrell,
Cancer 2010
N=343 • Cancro all’ovaio ESA
vs
No ESA
• Analisi retrospettiva
• Le pazienti trattate con ESA avevano fattori prognostici
sfavorevoli (più anziani, stadio più avanzato di malattia, più
ipertese)
• Nessuna differenza statisticamente significativa:
- Sopravvivenza globale
OR=0.851 p=0.35
- Sopravvivenza libera da progressione malattia
OR=0.959 p=0.488
Blohmer,
JCO 2011
N=257 • Carcinoma cervicale in stadio
non avanzato
• Target Hb: 12,5-13.5 g/dl
ESA
vs
No ESA
• Nessuna differenza statisticamente significativa nella
sopravvivenza globale
HR: 0.88 [95% CI, 0.51-1.50], p=0,63
Stehman F,
Gynecologic Oncology
2012
N=1864 • Cancro ovarico epiteliale, alle
tube di falloppio o primario
peritoneale dopo chirurgia
• Stadio avanzato
ESA
vs
No ESA
• Nessuna differenza statisticamente significativa:
- Sopravvivenza globale
HR=0,989 [IC95% 0,849–1,15] p=0.892
- Sopravvivenza libera da progressione malattia
HR=1,06 [IC 95% 0,937–1,19] p=0.364
Studi positivi](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-46-320.jpg)
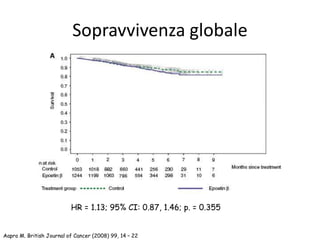
![Tonia, The Cochrane Collaboration 2012
Metanalisi Cochrane 2012
Sopravvivenza globale e mortalità
78 trials – 19.003 pazienti
Sopravvivenza globale: HR 1.05 [95% CI, 1.00-1.11]
Mortalità in studio: HR 1.17 [95% CI, 1.06-1.29]](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-48-320.jpg)
![Tonia, The Cochrane Collaboration 2012
Metanalisi Cochrane 2012
Riduzione del fabbisogno trasfusionale
57 trials – 15.877 pazienti
Riduzione fabbisogno trasfusionale: HR 0.65 [95% CI, 0.62-0.68]](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-49-320.jpg)
![OS sulla base dei livelli di Hb al basale
Se Hb basale<10 g/dl
HR=1.06 [95% CI, 0.96-1.17]
.
.
.
.
.
.
Tonia, The Cochrane Collaboration 2012](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-50-320.jpg)
![OS sulla base dei livelli di Hb al basale
Se Hb basale 10-12 g/dl
HR=1.01 [95% CI, 0.93-1.10]
.
.
.
.
.
.
Tonia, The Cochrane Collaboration 2012](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-51-320.jpg)
![OS sulla base dei livelli di Hb al basale
Se Hb basale >12 g/dl
HR=1.17 [95% CI, 1.06-1.29]
Tonia, The Cochrane Collaboration 2012](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-52-320.jpg)
![OS sulla base delle diverse terapie
Se pazieti in trattamento CHT
HR=1.04 [95% CI, 0.98-1.11]
Tonia, The Cochrane Collaboration 2012](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-53-320.jpg)
![OS sulla base delle diverse terapie
Se pazienti non in trattamento (RT/CHT)
HR=1.23 [95% CI, 1.04-1.45]
Tonia, The Cochrane Collaboration 2012](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-54-320.jpg)
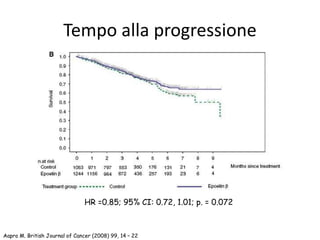
![Tonia, The Cochrane Collaboration 2012
Metanalisi Cochrane 2012
Rischio di eventi tromboembolici
57 trials – 15.278 pazienti
TVE: HR 1.52 [95% CI, 1.33-1.73]](https://image.slidesharecdn.com/11-150114074906-conversion-gate01/85/Anemo-2014-Lorusso-ESA-nel-paziente-oncologico-57-320.jpg)