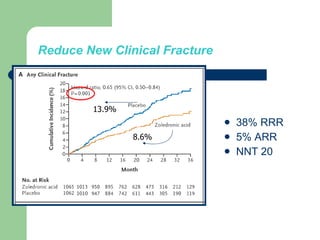
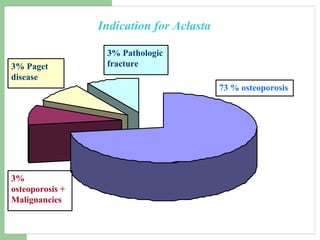
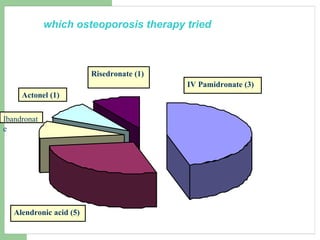
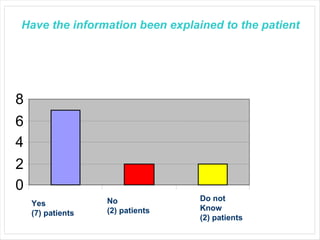
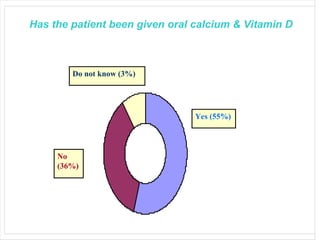
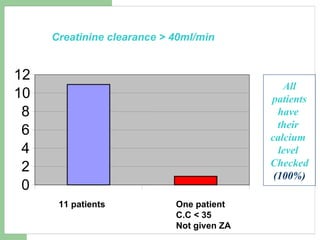
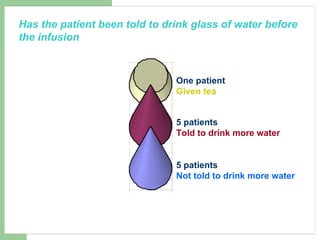
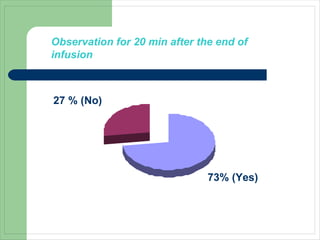
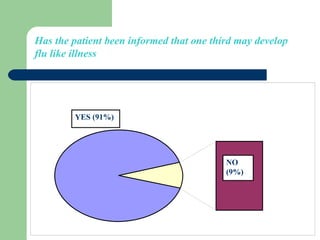
This audit summarizes the current practice of zoledronic acid therapy at NNUH. It found that 73% of patients received zoledronic acid for osteoporosis. Most patients had previously tried other osteoporosis therapies. While most patients received information and supplements, some did not have information fully explained or receive calcium and vitamin D. All patients had creatinine clearance and calcium levels checked. Most were told to drink water before and observed after the infusion, though a few were not. The audit concluded areas of current practice that could be improved.