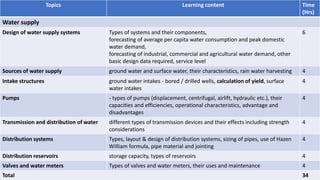
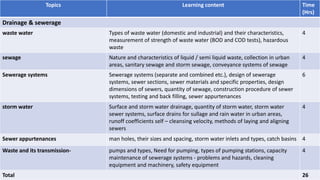
The document provides information about a module on water supply, drainage and sewerage technology. The module aims to enable students to determine capacity requirements and supervise operation and maintenance of these systems. It includes topics like water supply design, sources of water, pumps, transmission and distribution systems. For drainage and sewerage, it covers waste water characteristics, sewerage systems design, and storm water drainage. The document also outlines the learning outcomes, lecture hours, assessment methods and topics to be covered in the module.