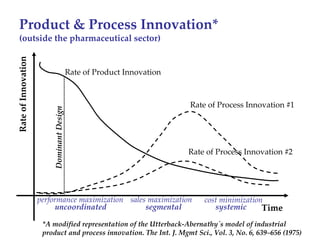
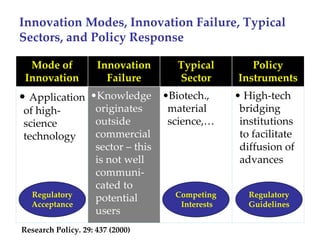
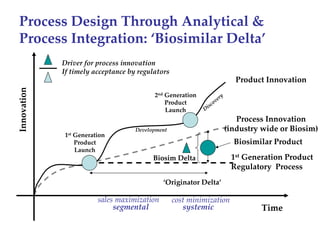
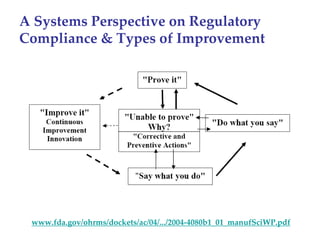
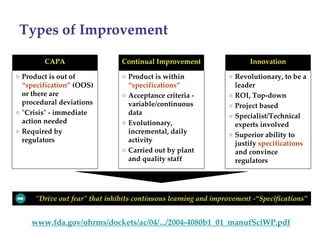
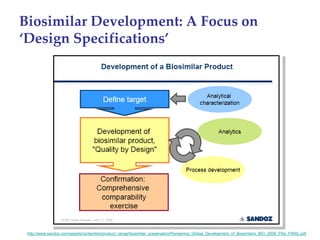
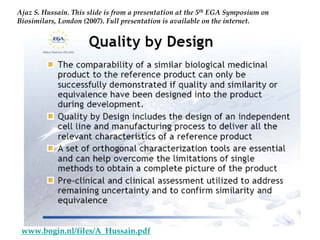
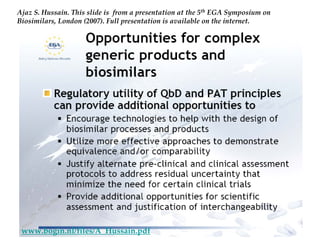
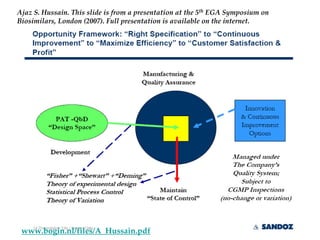
The keynote address discusses whether biosimilars will drive innovation in biopharmaceutical manufacturing, concluding that while competition may foster product innovation, it is not expected to drive process innovation due to significant regulatory barriers. The speaker emphasizes that systemic issues in communication and regulatory acceptance contribute to innovation failure in a highly regulated environment. The biosimilars debate highlights these failures, underscoring the need for effective scientific resolutions within the sector.