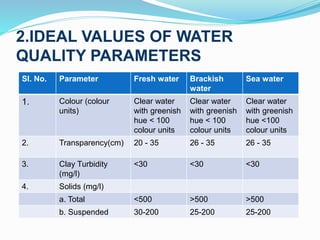
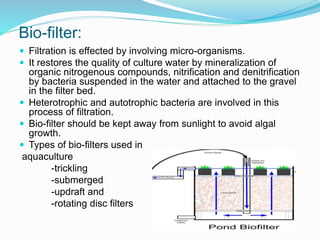
This document provides information on aquaculture environment management and water quality management. It discusses analyzing water quality parameters, understanding ideal value ranges, and using chemical treatment and mechanical control. Specific parameters like nitrogen, pH, dissolved oxygen are analyzed. Common treatments include liming to adjust pH, using alum as a coagulant, and chlorination for disinfection. Filtration through gravel or activated carbon can be used for mechanical control. The objective is to manage water quality to provide optimal growing conditions for farmed fish.