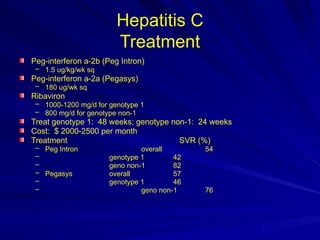
The document outlines the history, transmission, clinical features, and prevention of viral hepatitis types A to E. It includes details on epidemiology, diagnosis, and treatment approaches for each type, emphasizing their distinct characteristics and transmission routes. Additionally, the document mentions the challenges posed by chronic infections and the importance of vaccination and immunoprophylaxis.