Embed presentation
Download to read offline




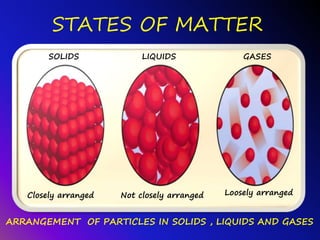


Matter can be classified into three states - solids, liquids, and gases. Solids have a definite shape and volume, while liquids have a definite volume but no definite shape. Gases have neither a definite shape nor volume. The key differences between the three states are the arrangement and movement of their particles - solid particles are closely packed, liquid particles are less closely packed, and gas particles are loosely arranged and flow freely in all directions.






