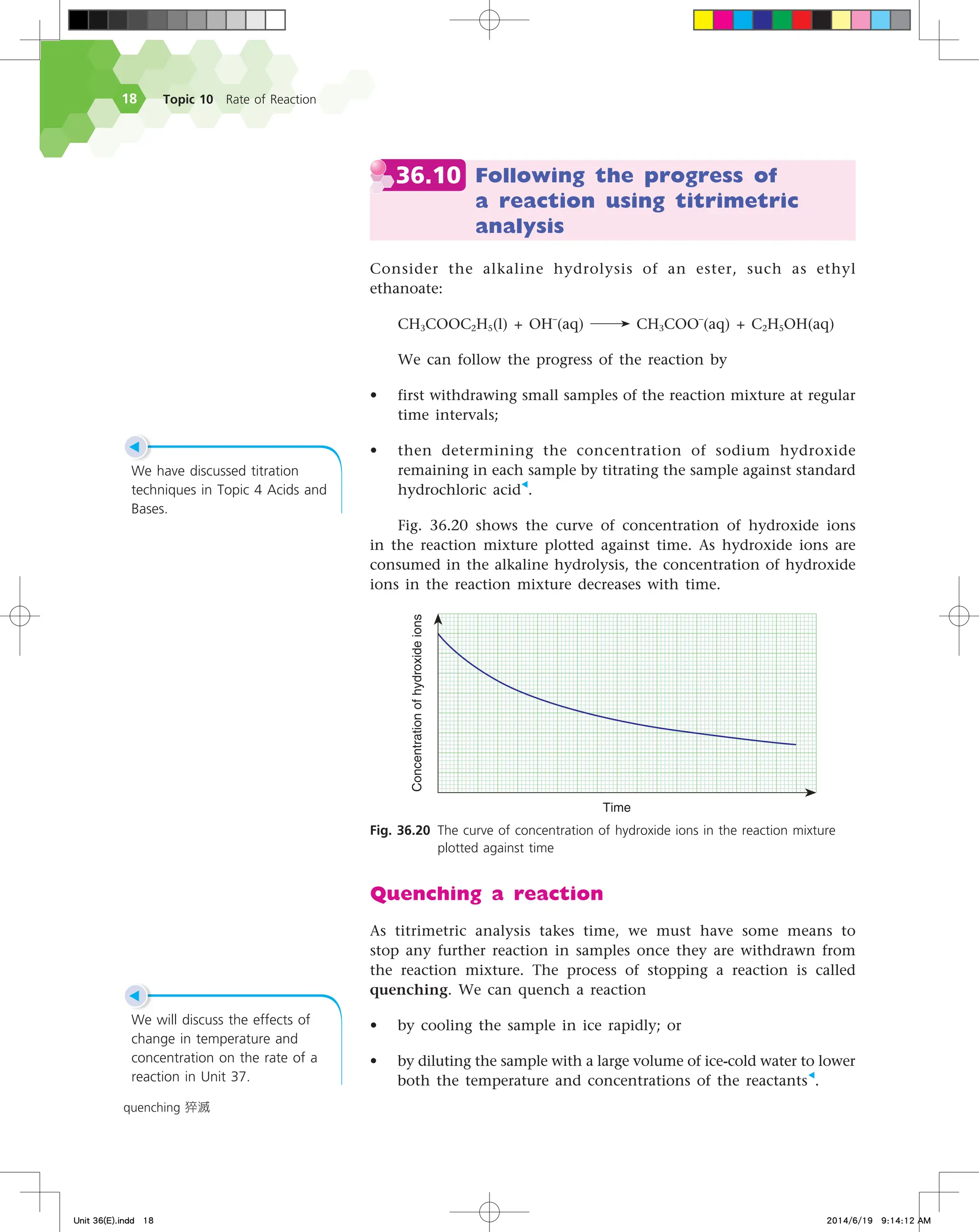
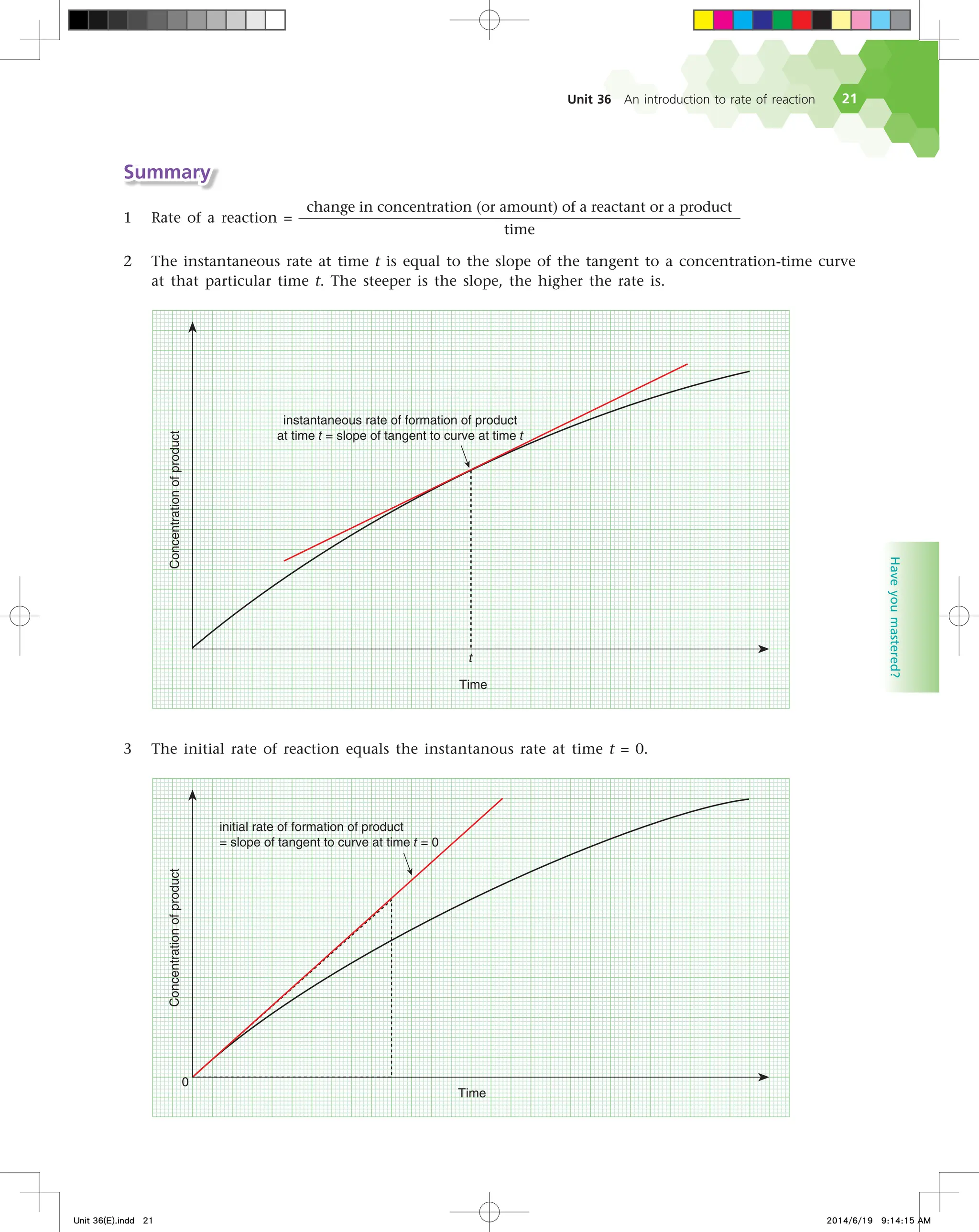
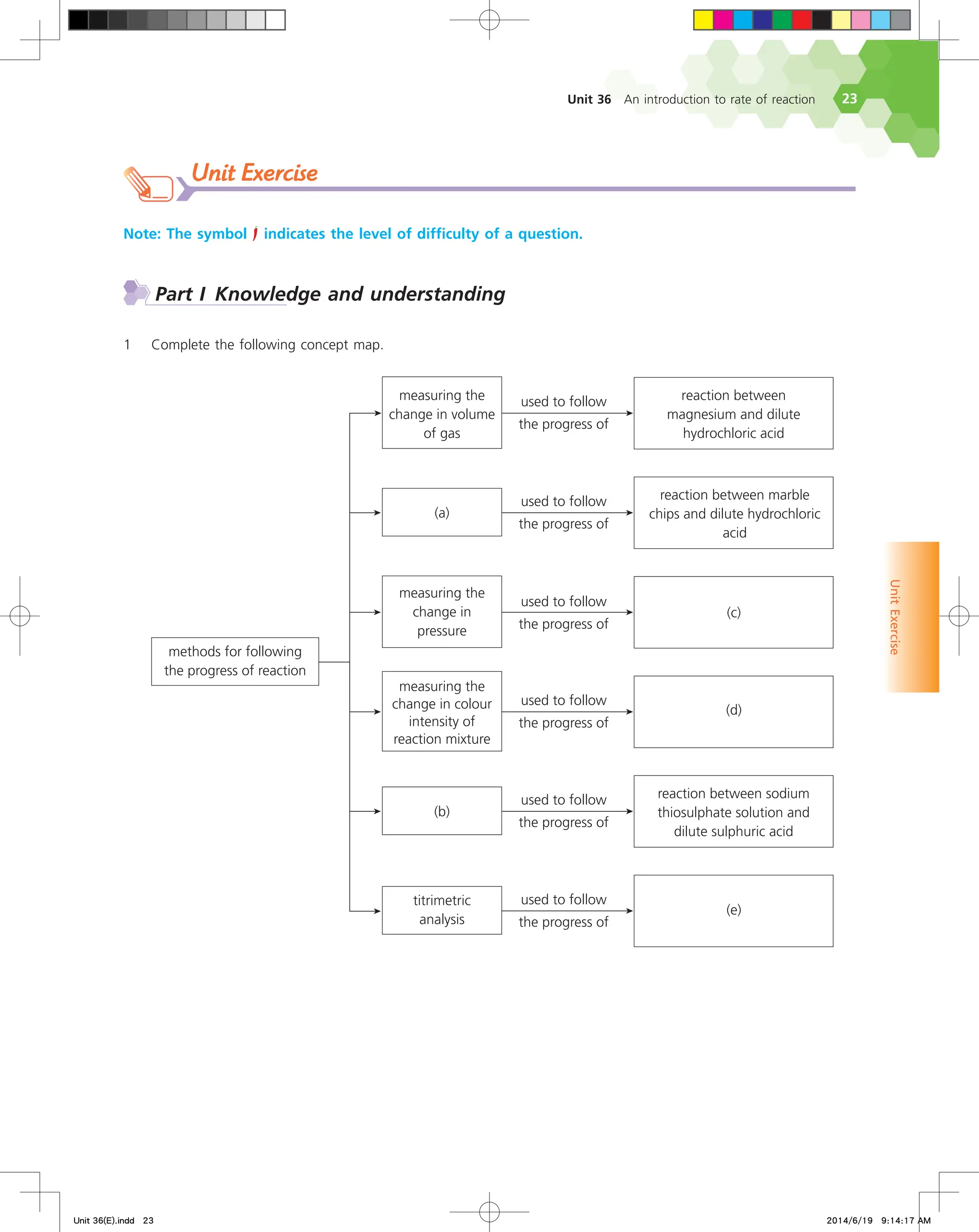
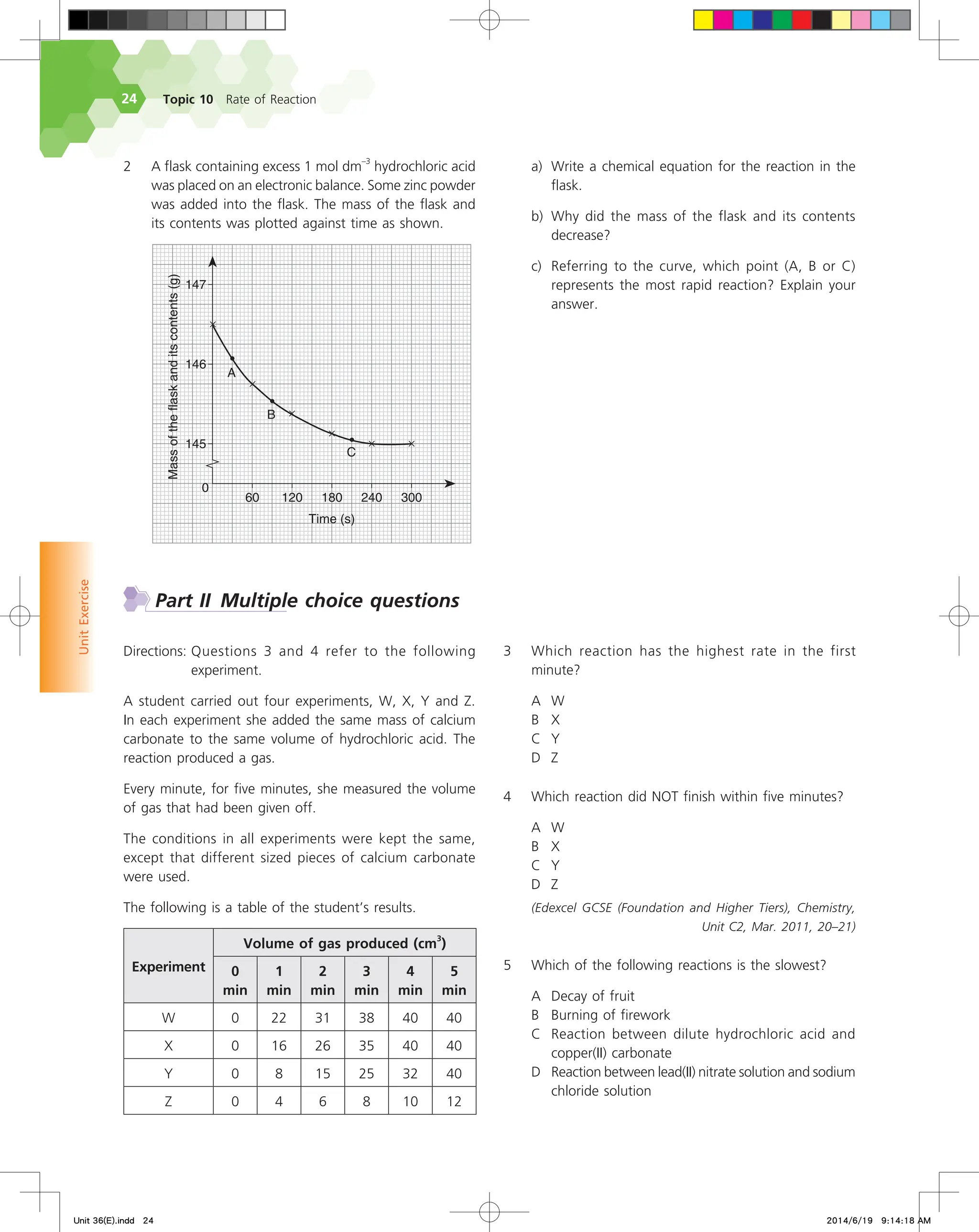
The document discusses fire extinguishers that use a chemical reaction to extinguish fires. Specifically, it describes soda-acid fire extinguishers, which contain a bottle of sulfuric acid and a container of sodium bicarbonate solution. When the extinguisher is activated, the acid and solution mix and react, producing carbon dioxide gas. This pressurizes the water inside and forces it out to extinguish the fire. The reaction between the sodium bicarbonate solution and sulfuric acid produces carbon dioxide and water, pressurizing the extinguisher.


![Topic 10 Rate of Reaction
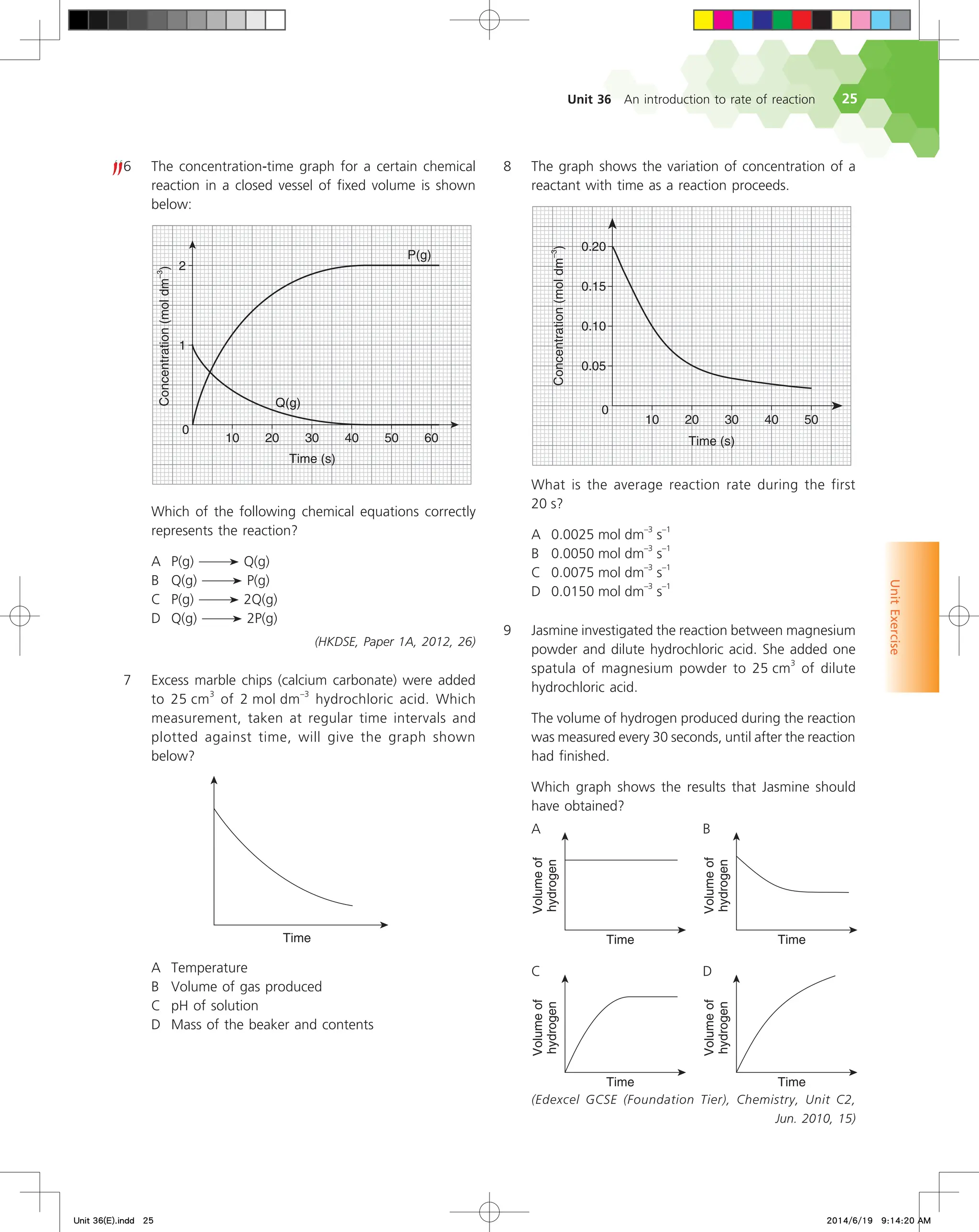
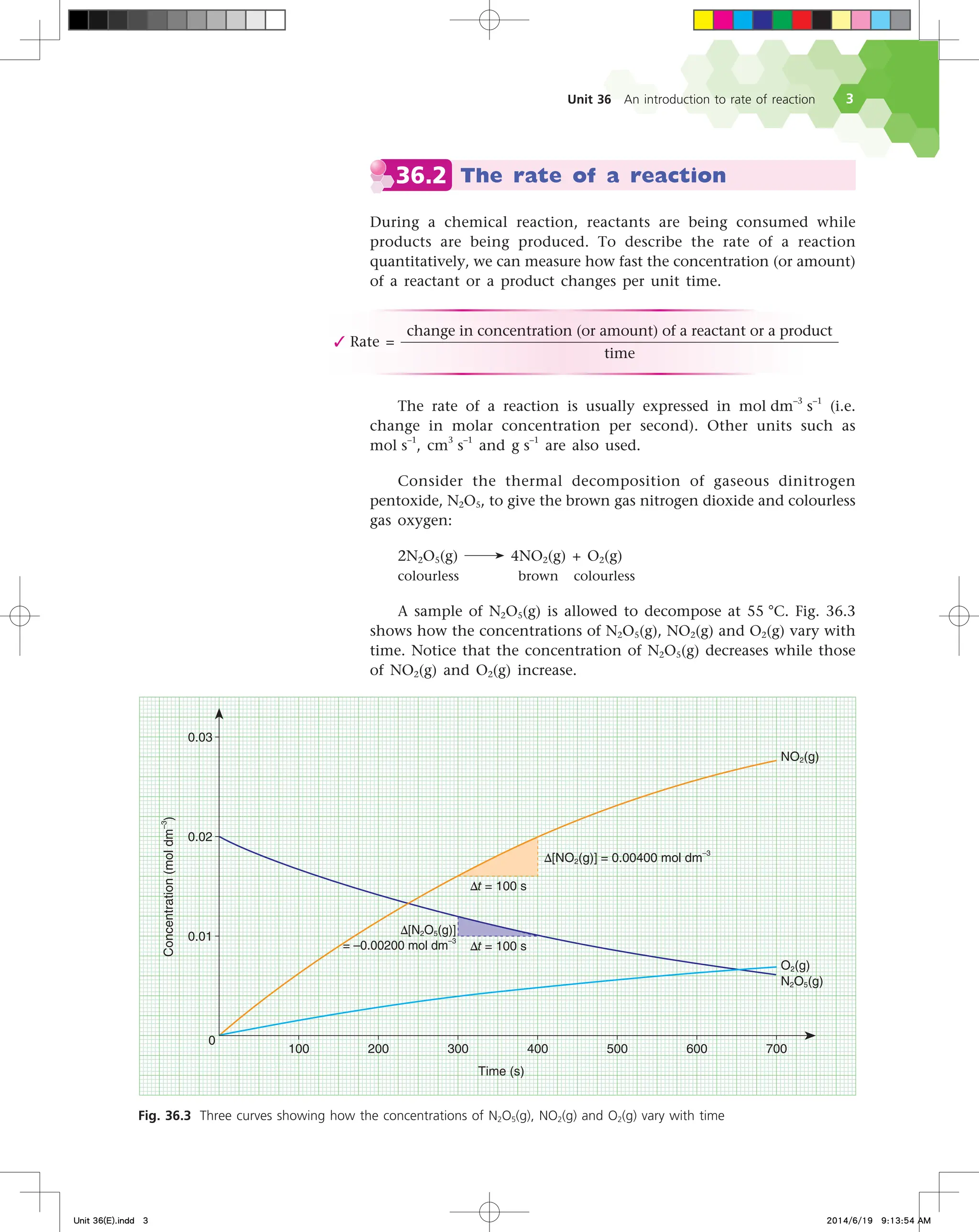
We can define the rate of a reaction either as the increase in
the concentration of a product per unit time or the decrease in the
concentration of a reactant per unit time.
Let us first look at the formation of nitrogen dioxide. The rate
of formation of nitrogen dioxide is given by the expression:
Rate of formation of NO2(g)
=
concentration of NO2(g) at time t2 – concentration of NO2(g) at time t1
t2 – t1
=
∆[NO2(g)]
∆t
In the above expression,
• square brackets surrounding NO2(g) denote its concentration in
mol dm–3
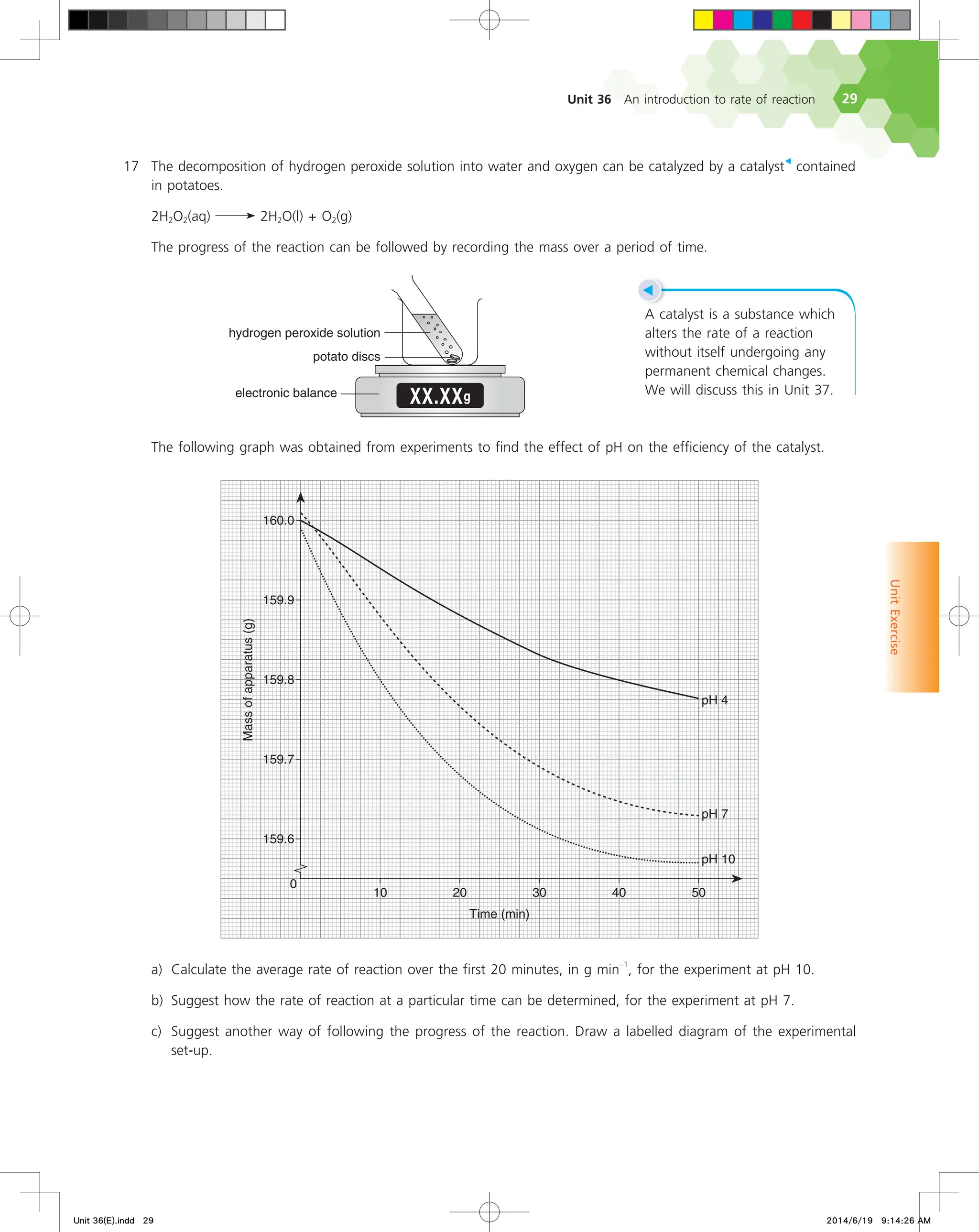
, and ∆[NO2(g)] is the change in concentration of NO2(g)
during the interval from t1 to t2;
• ∆t is the change in time.
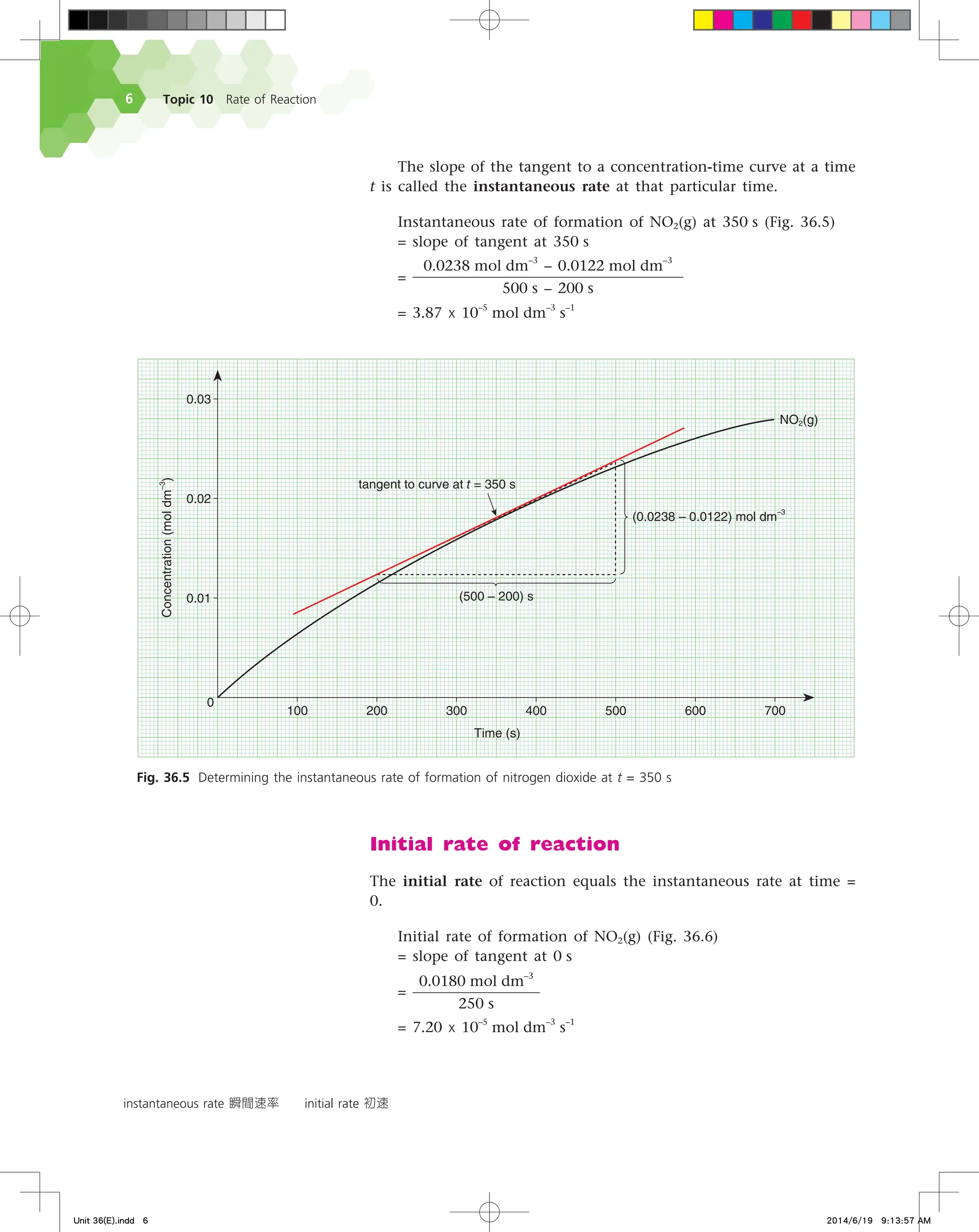
Refer to Fig. 36.3 again. Look at the triangle drawn on the
curve for nitrogen dioxide between the time period 300 s to 400 s.
∆[NO2(g)] represents the vertical side of the triangle while ∆t represents
the horizontal side. The slope of the hypotenuse of the triangle is
∆[NO2(g)]
∆t
, the average rate of formation of nitrogen dioxide during
that time period.
Average rate of formation of NO2(g)
=
∆[NO2(g)]
∆t
=
0.0200 mol dm
–3
– 0.0160 mol dm
–3
400 s – 300 s
= 4.00 x 10–5
mol dm
–3
s
–1
Now look at the triangle drawn on the curve for dinitrogen
pentoxide. It is defined by ∆[N2O5(g)] and ∆t. As the concentration
of dinitrogen pentoxide decreases with time,
∆[N2O5(g)]
∆t
is a negative
quantity.
As it is usual to work with positive reaction rates, we always
introduce a minus sign when calculating the rate of disappearance
of a reactant. We calculate the average rate of decomposition of
dinitrogen pentoxide during the 300 s to 400 s period as follows:
slope 斜率 average rate 平均速率
Unit 36(E).indd 4 2014/6/19 9:13:55 AM](https://image.slidesharecdn.com/unit36e-231118065451-9e342fc5/75/Unit-36-E-pdf-4-2048.jpg)
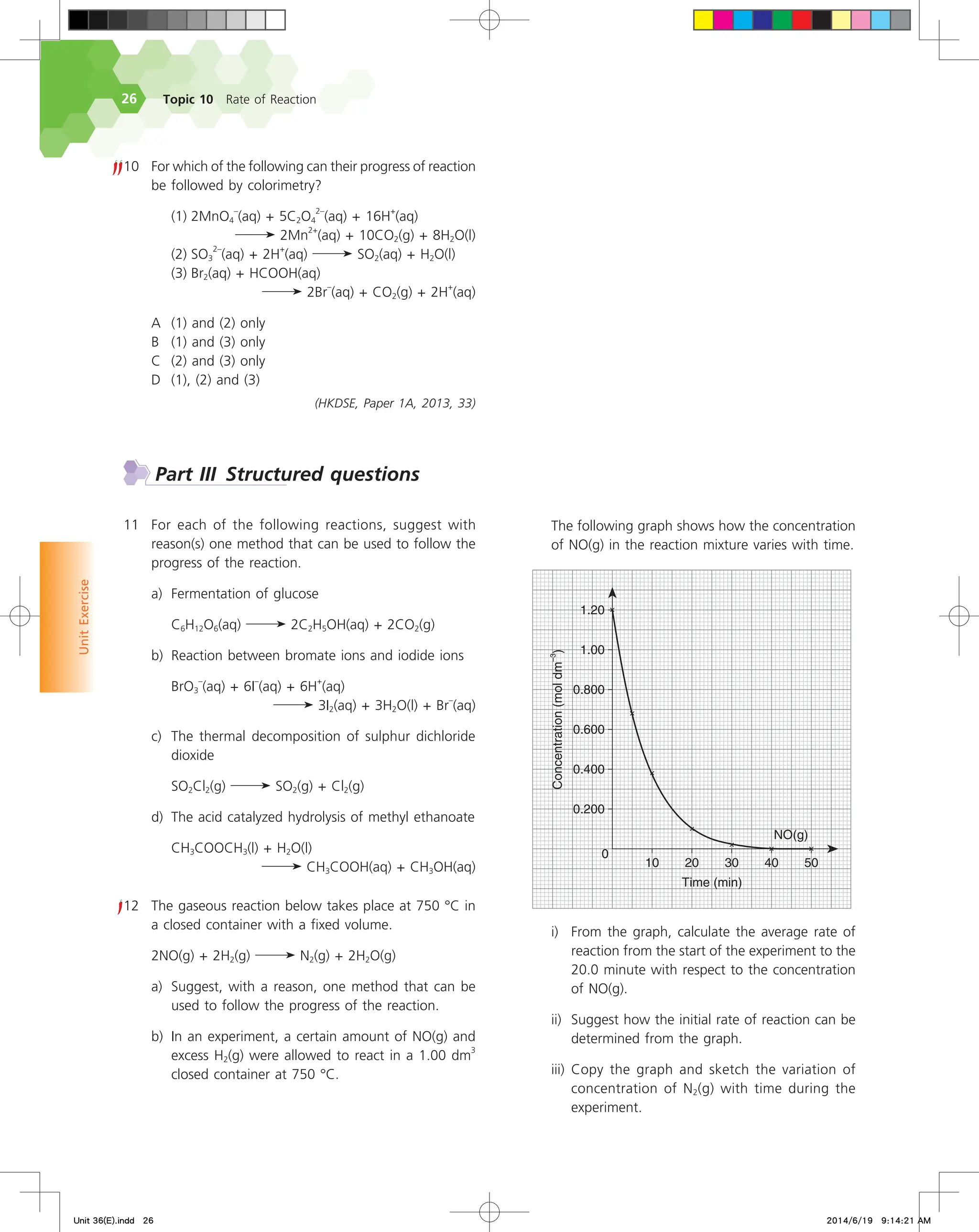
![Unit 36 An introduction to rate of reaction
Average rate of decomposition of N2O5(g)
= –
∆[N2O5(g)]
∆t
= –
0.0100 mol dm
–3
– 0.0120 mol dm
–3
400 s – 300 s
= 2.00 x 10
–5
mol dm
–3
s
–1
When quoting a reaction rate, it is important to specify the
reactant or product on which the rate is based because rates of
reactant disappearance and product formation may differ, as in this
example.
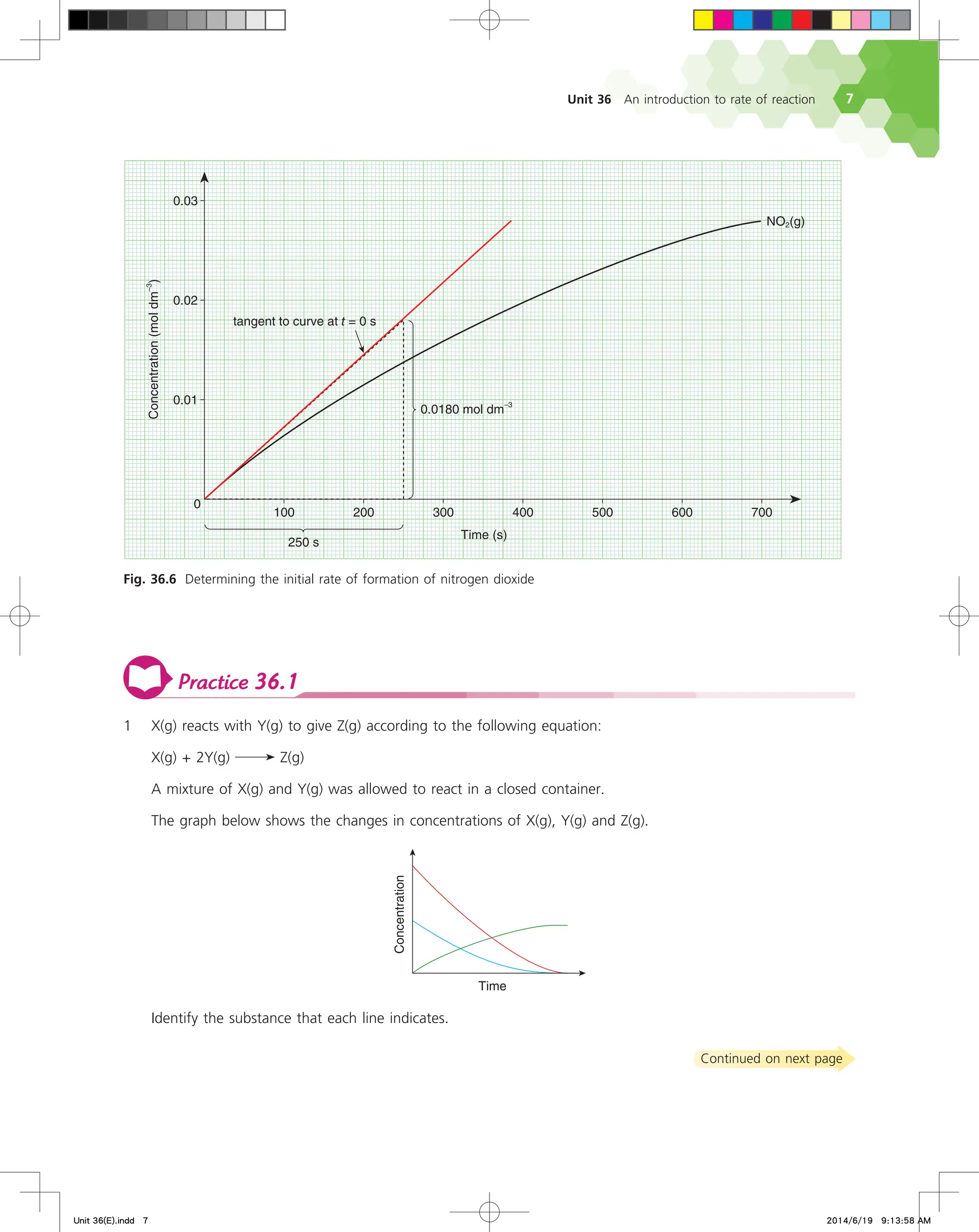
Instantaneous rate of reaction
36.3
Often, chemists want to know the rate of a reaction at a specific time
rather than the rate averaged over a time interval ∆t.
Refer to Fig. 36.4 that shows only the concentration of nitrogen
dioxide plotted against time when dinitrogen pentoxide decomposes
at 55 °C. If we make our measurements at shorter and shorter time
intervals, the triangle defined by ∆[NO2(g)] and ∆t will shrink to a
point, and the slope of the hypotenuse of the triangle will approach
the slope of the tangent to the curve at t = 350 s.
Fig. 36.4 The concentration of nitrogen dioxide plotted against time when dinitrogen pentoxide decomposes at 55 °C
5JNF T
/0 H
$PODFOUSBUJPO
NPMEN
m
UBOHFOUUPDVSWF
BUUT
tangent 切線
Unit 36(E).indd 5 2014/6/19 9:13:56 AM](https://image.slidesharecdn.com/unit36e-231118065451-9e342fc5/75/Unit-36-E-pdf-5-2048.jpg)