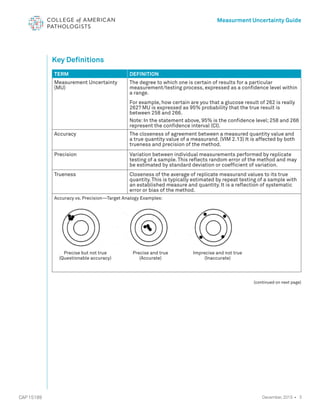
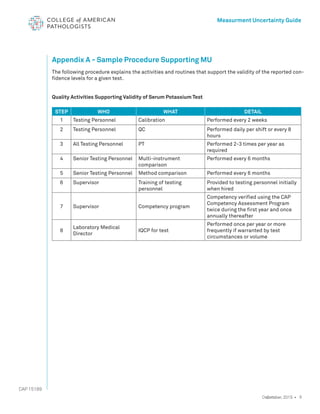
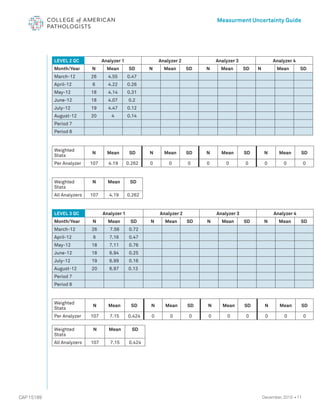
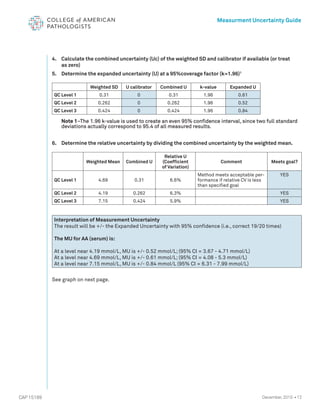
The document serves as a guide for measurement uncertainty (mu) in laboratories seeking ISO 15189 accreditation, emphasizing the need for laboratories to determine and provide measurement uncertainty for each test procedure. It outlines CAP's assessment standards, routine quality control practices, and guidelines for calculating and reporting mu, while noting that this does not apply to point-of-care testing. Key definitions related to accuracy, precision, and the implications of measurement uncertainty are also detailed to assist laboratories in compliance and best practices.