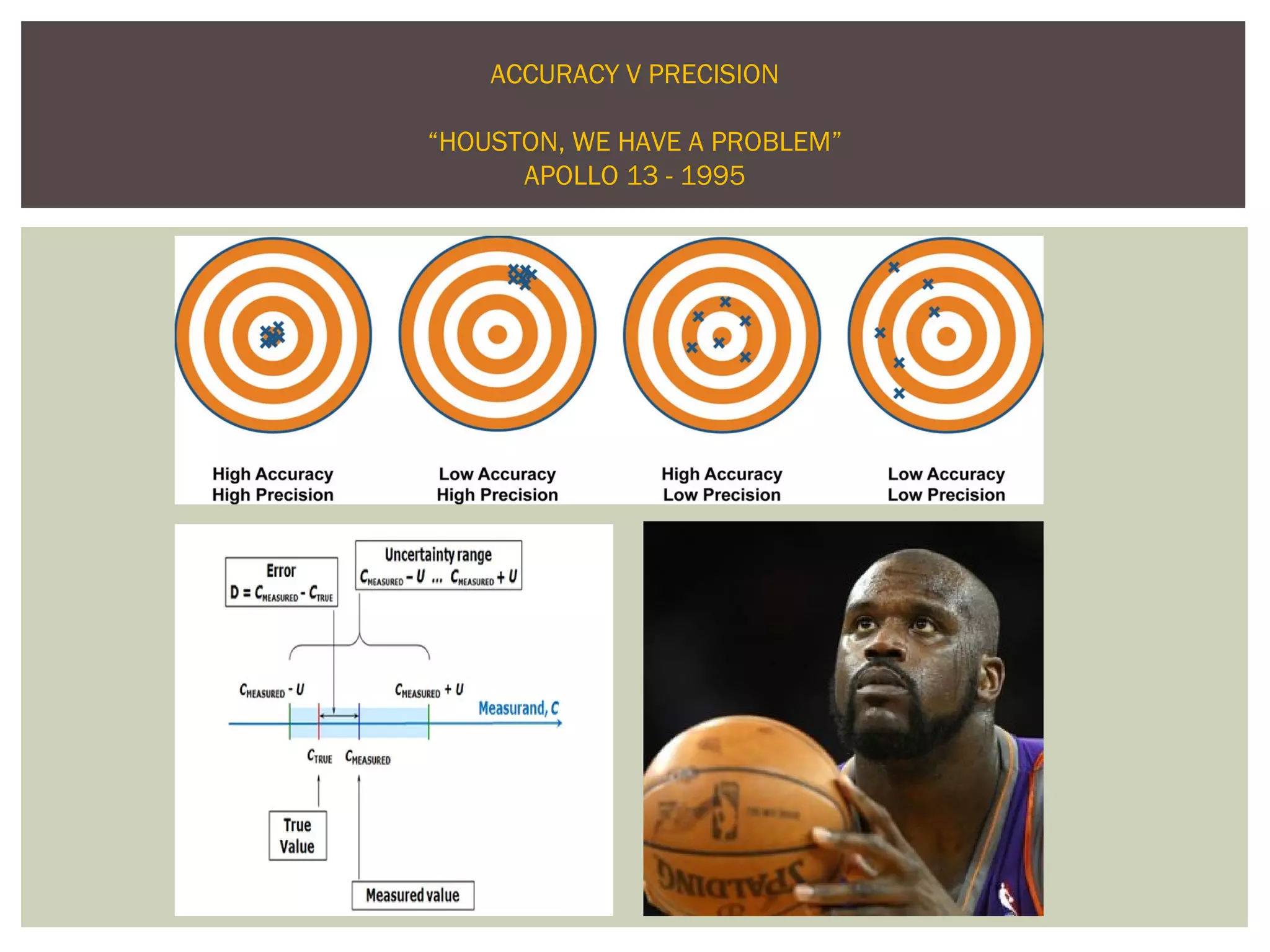
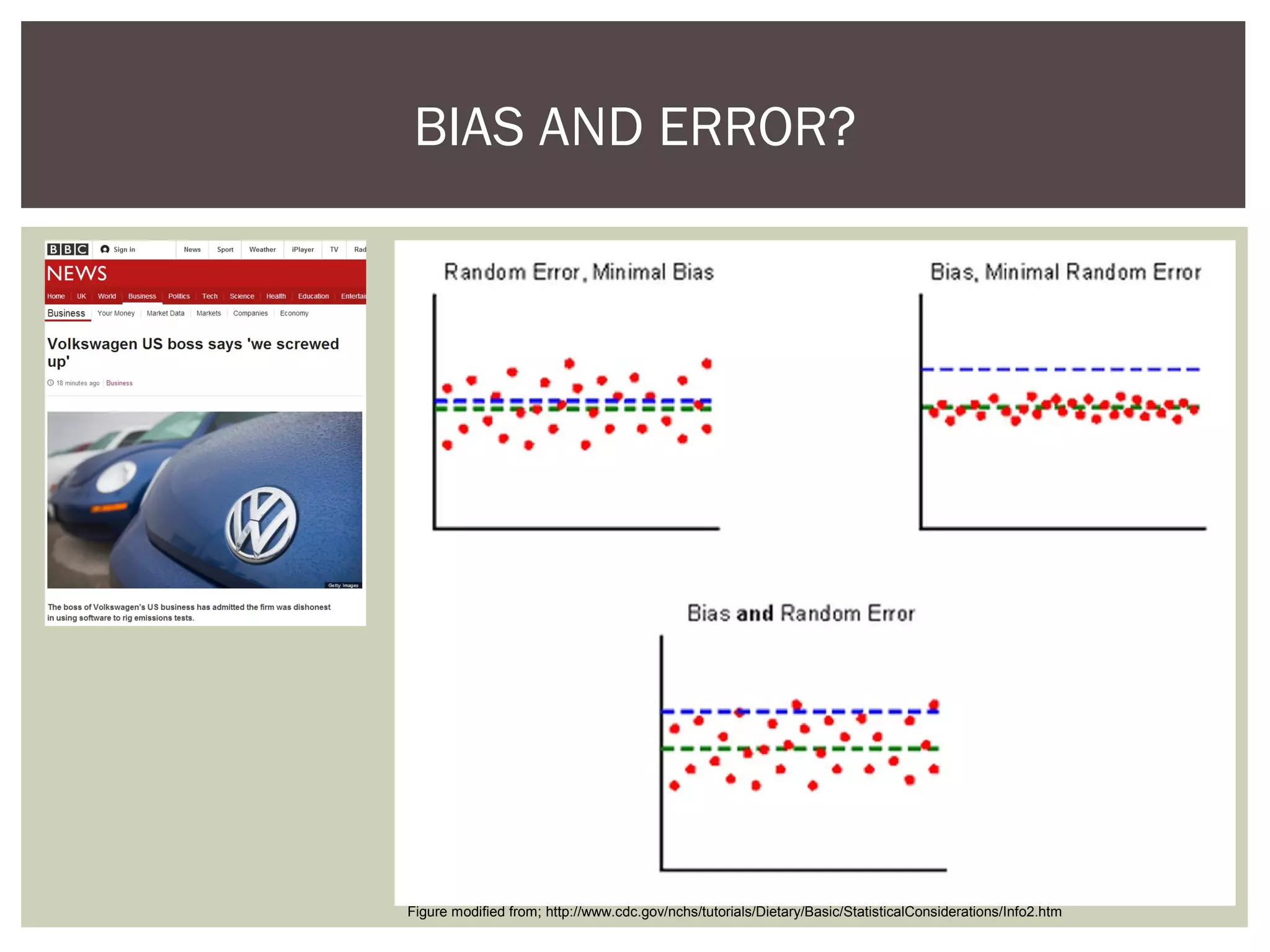
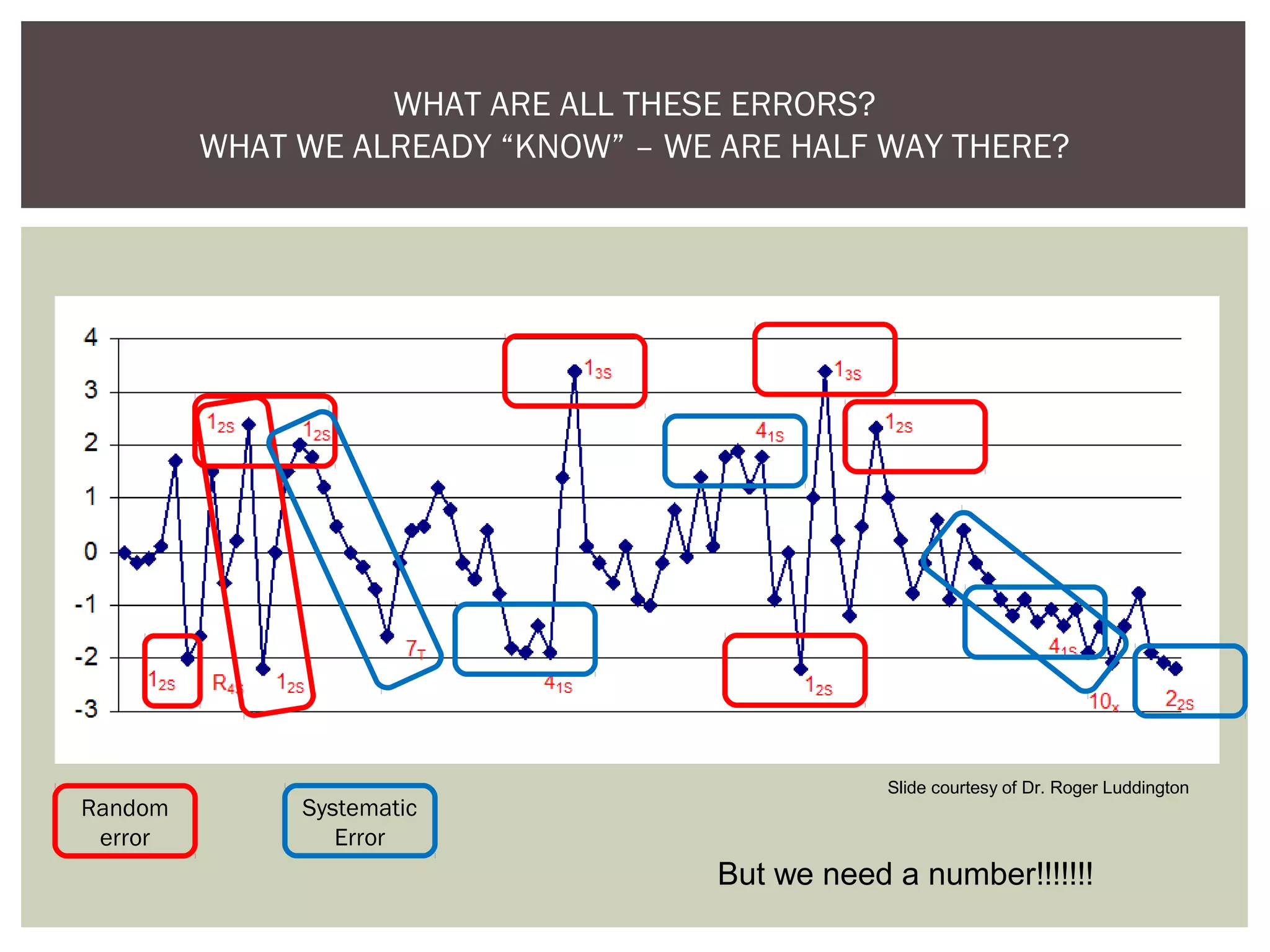
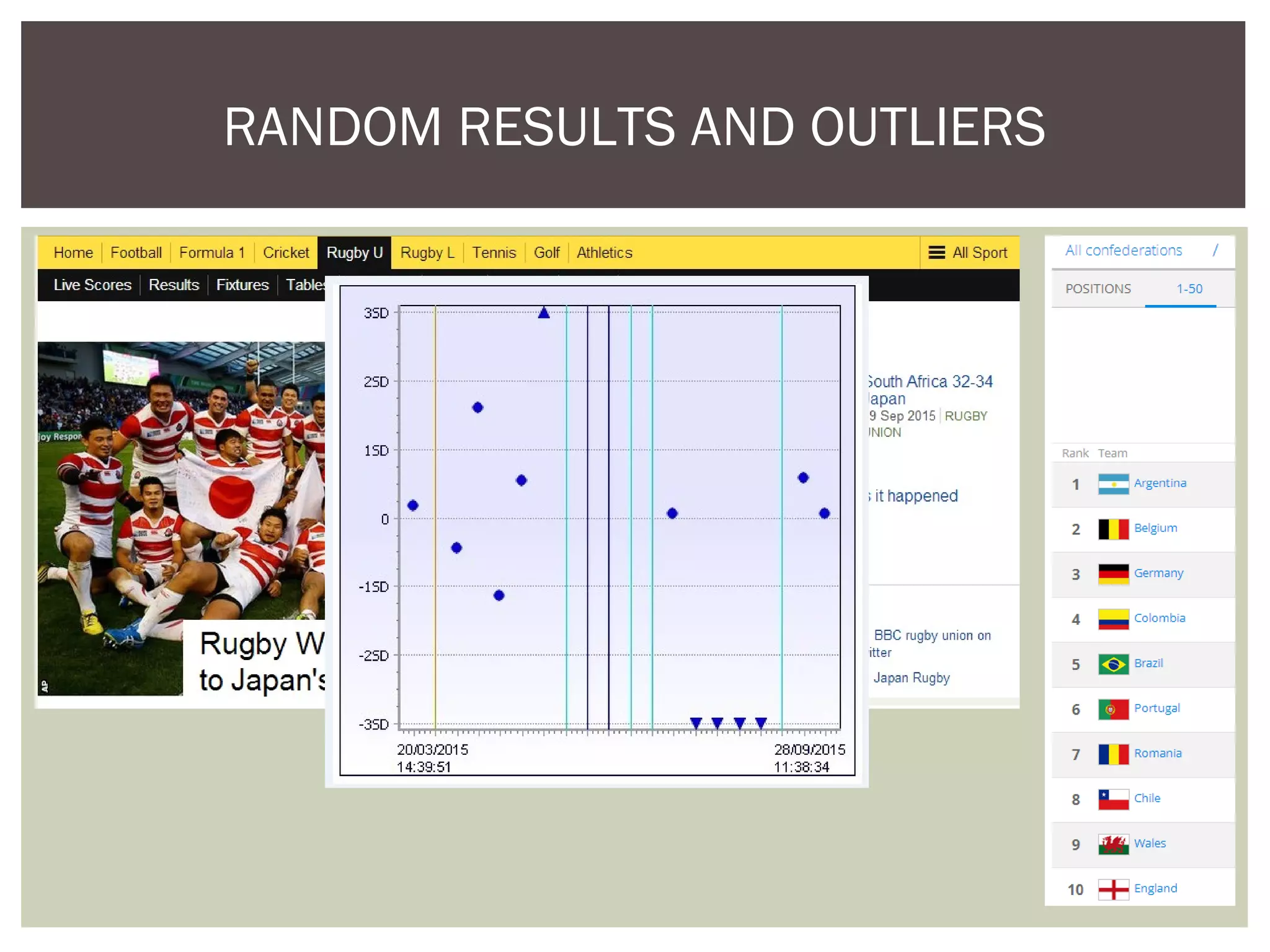
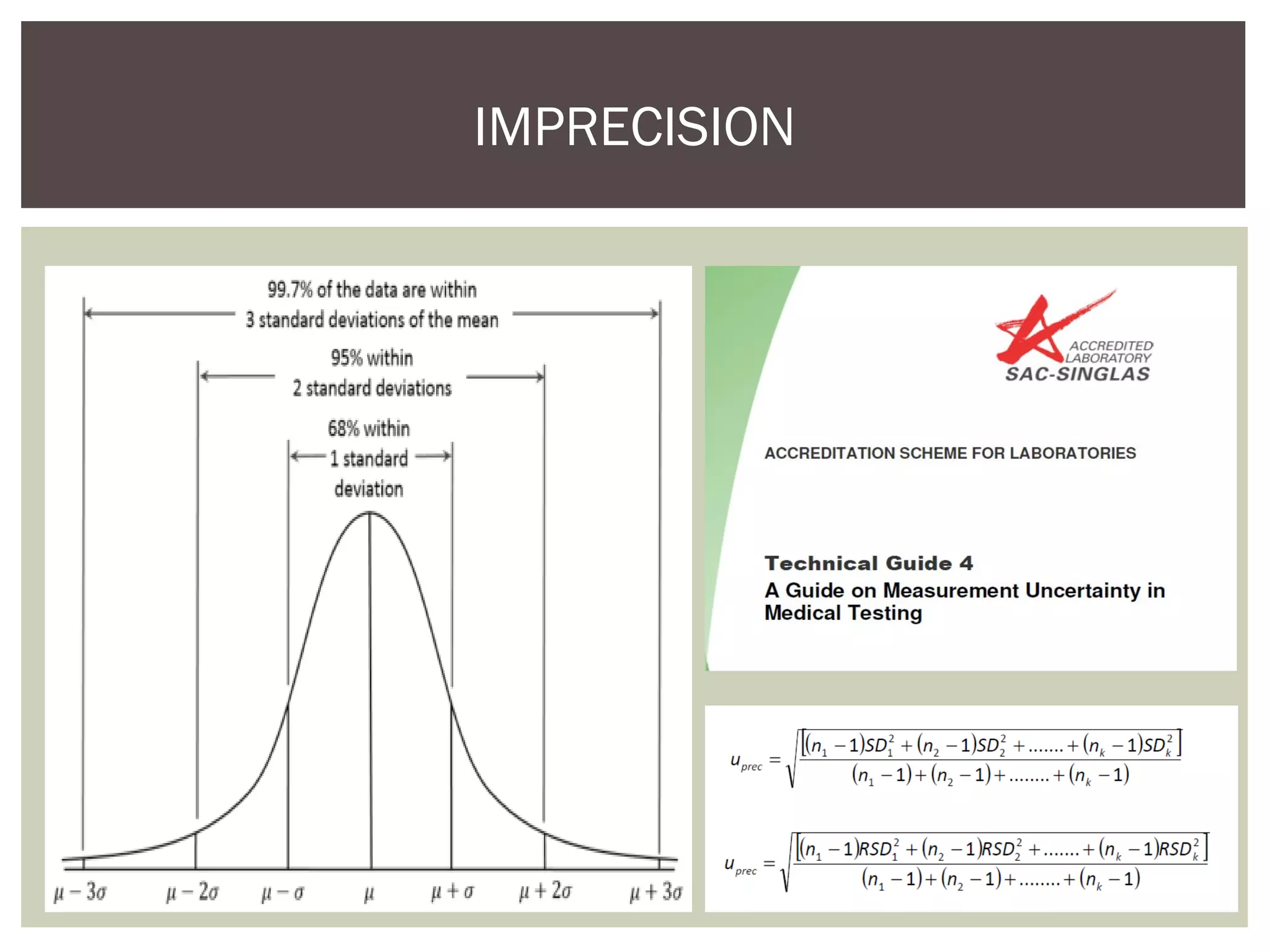
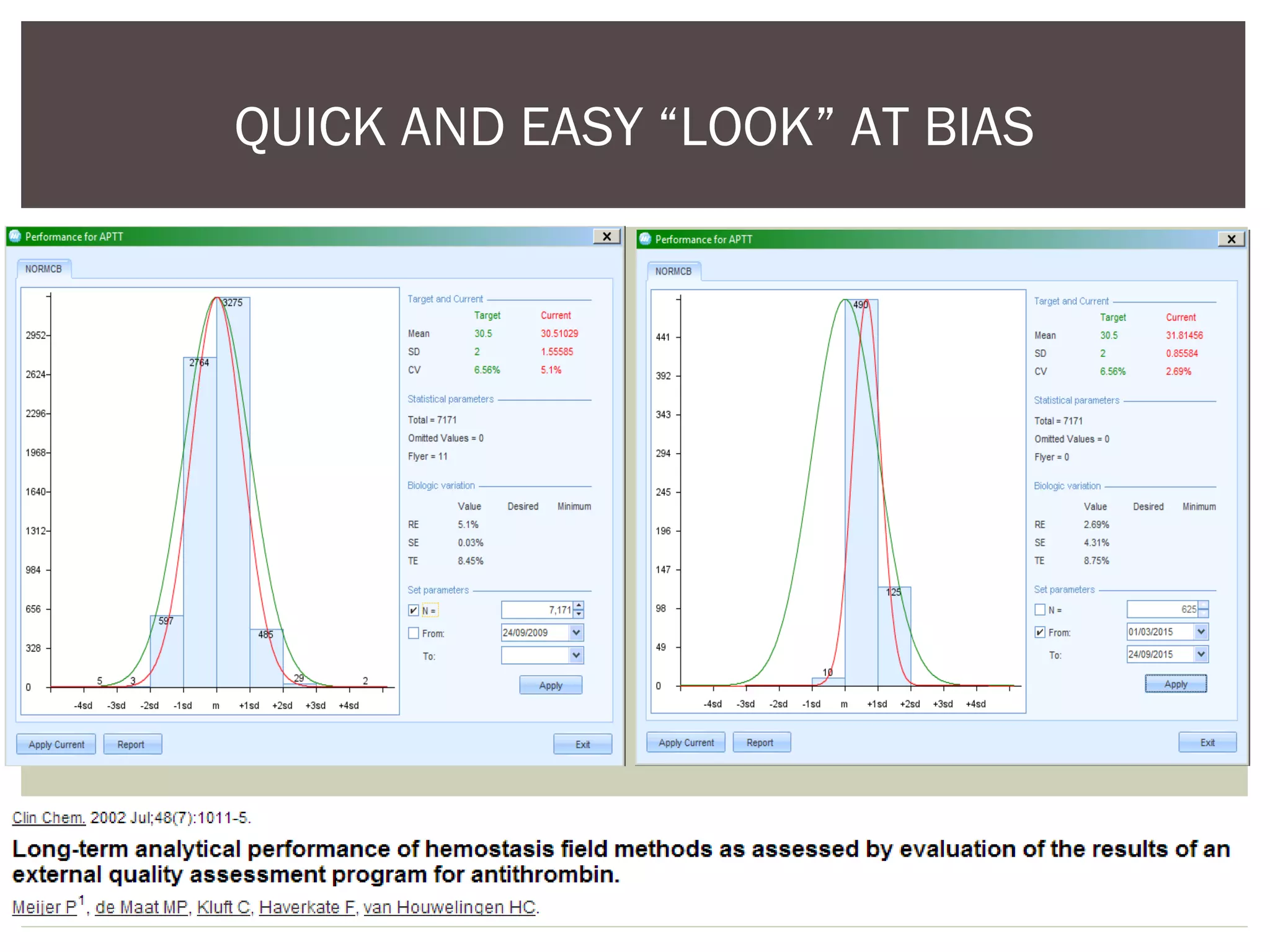
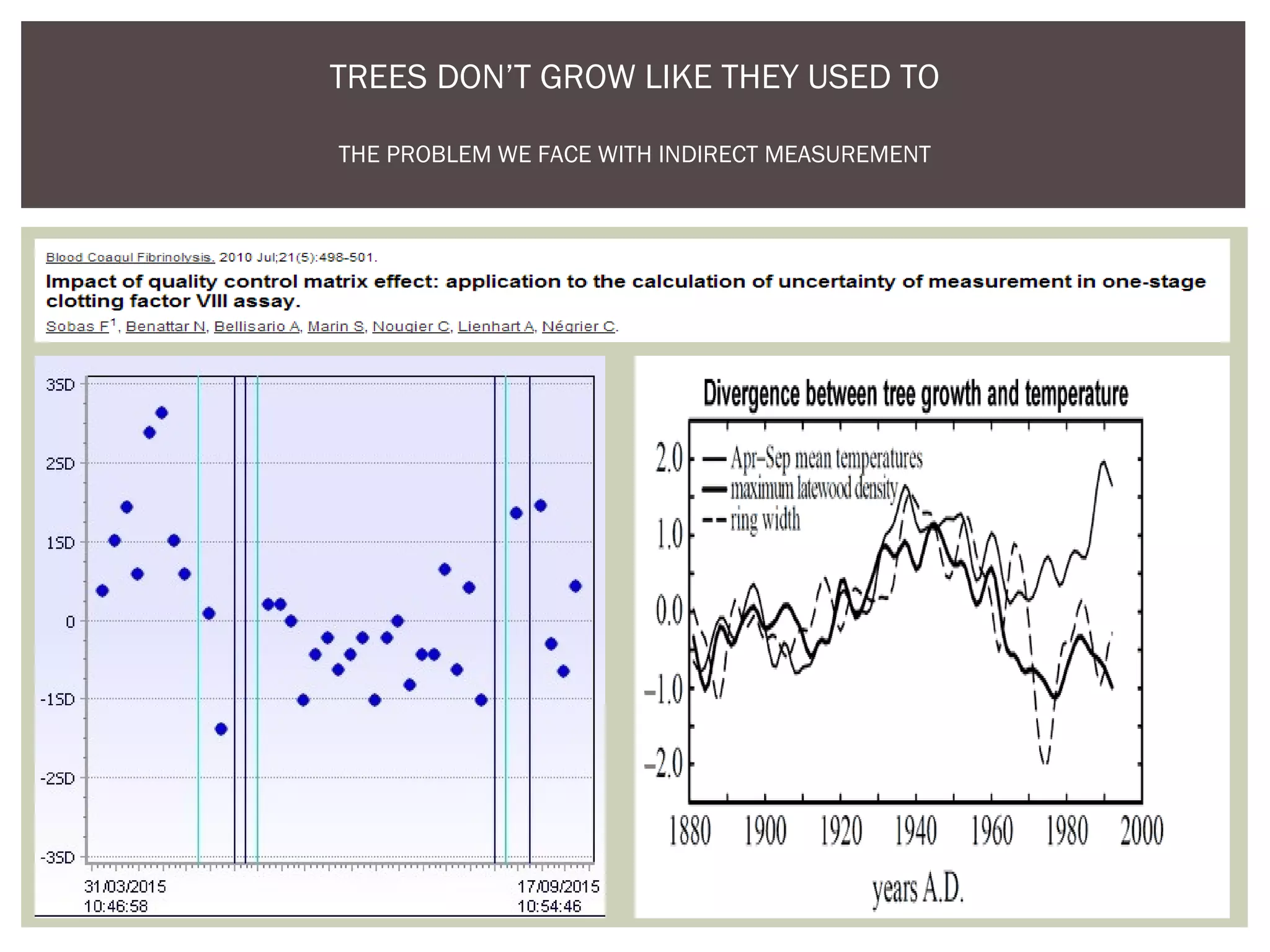
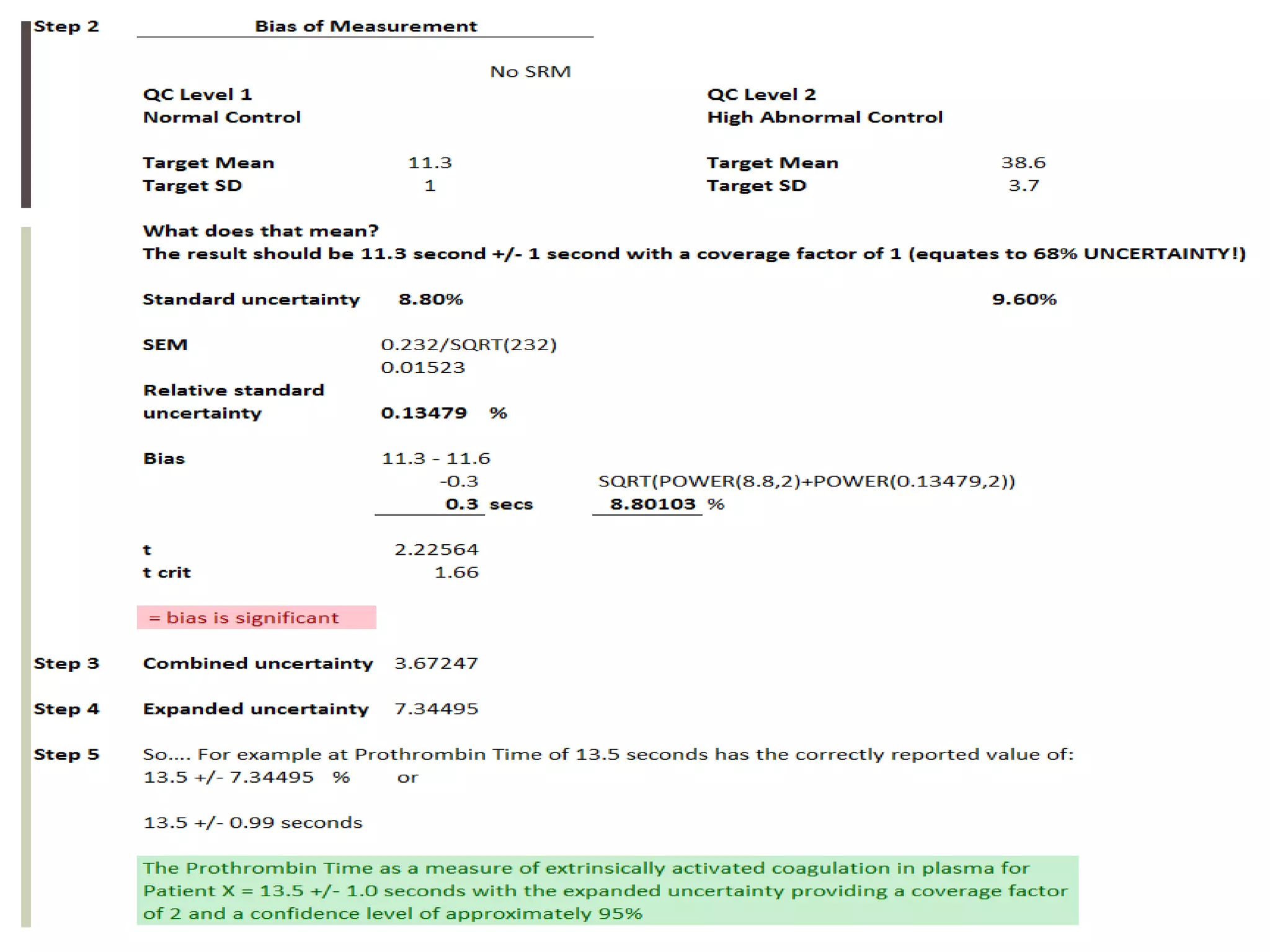
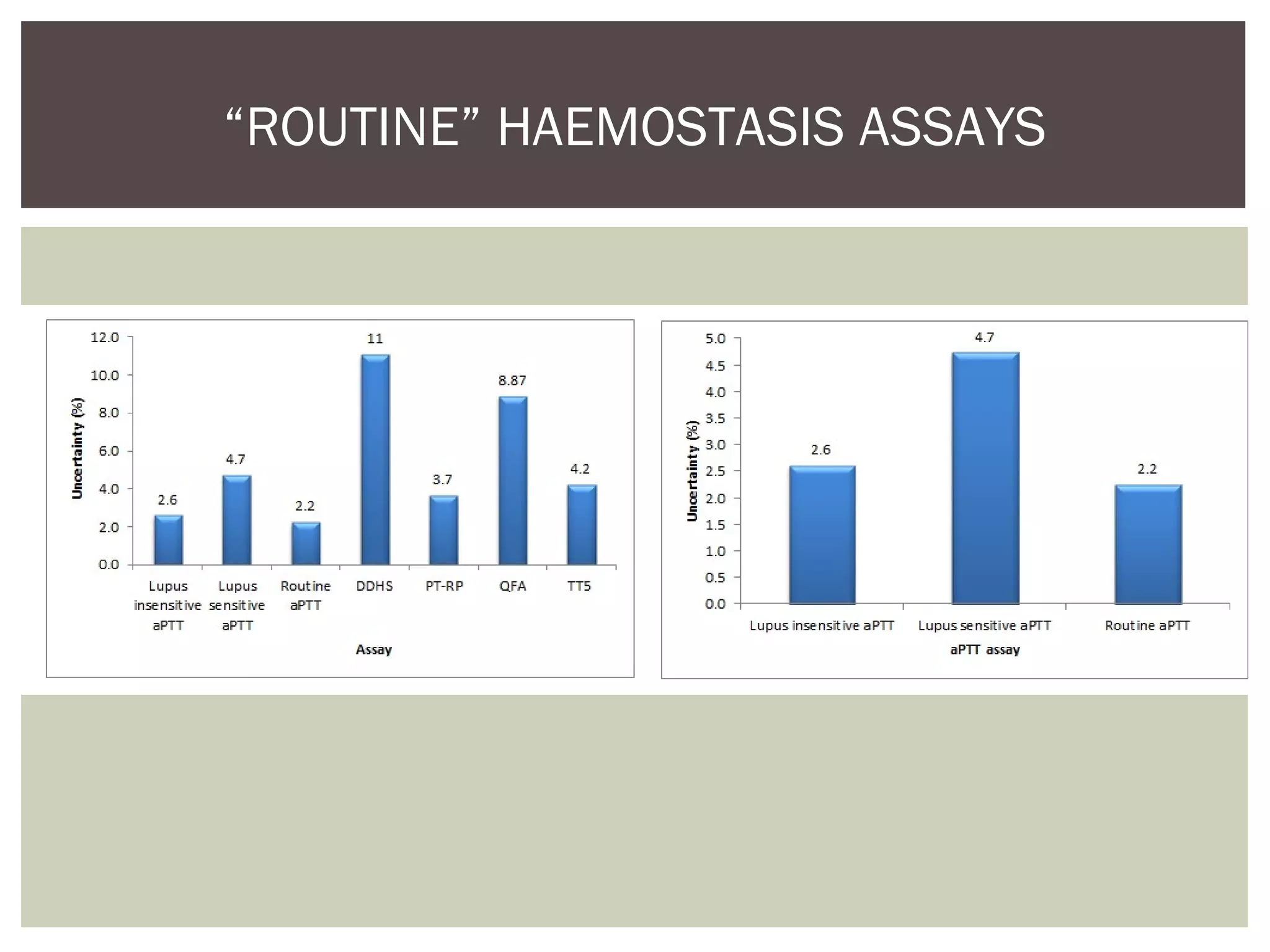
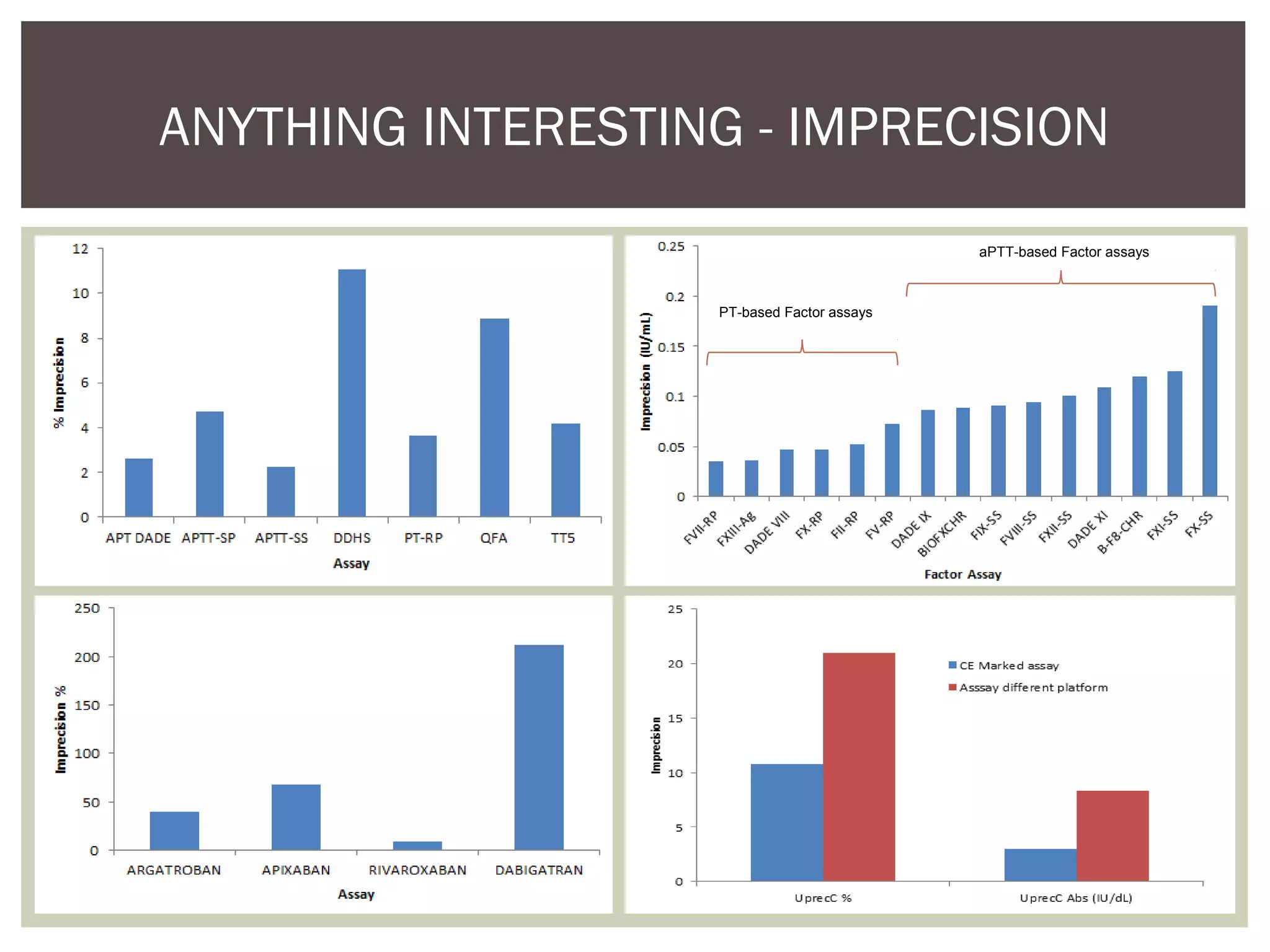
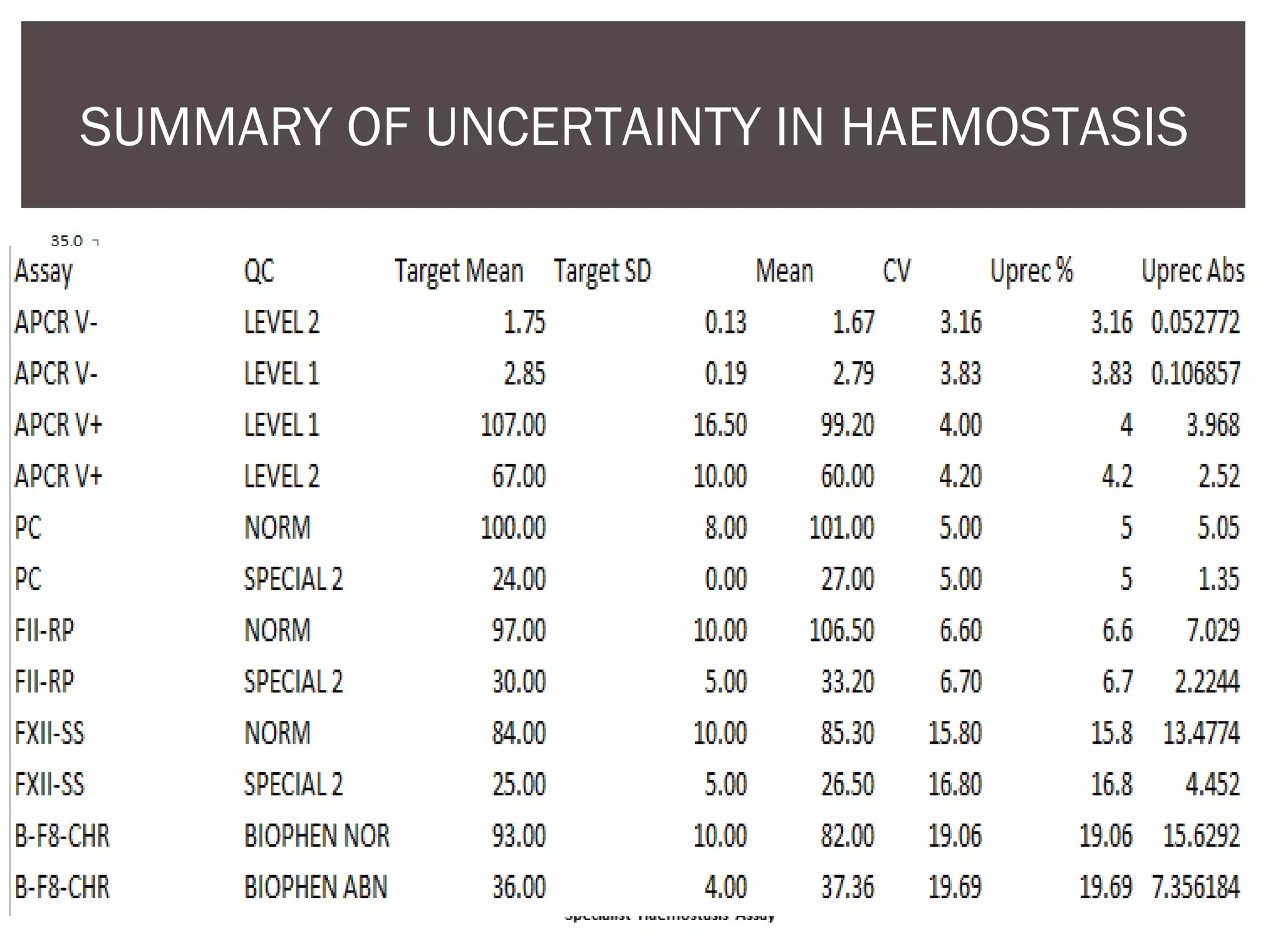
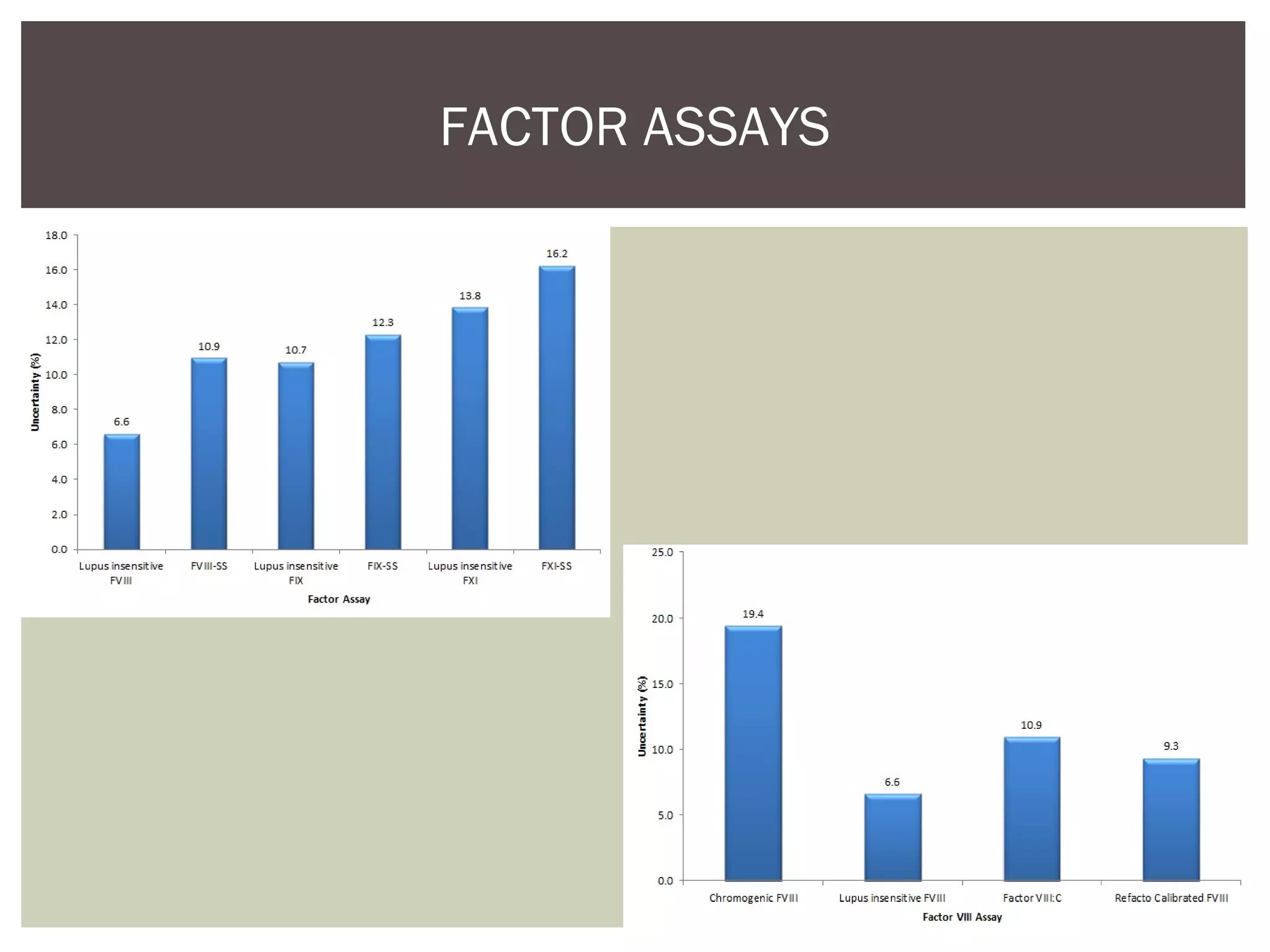
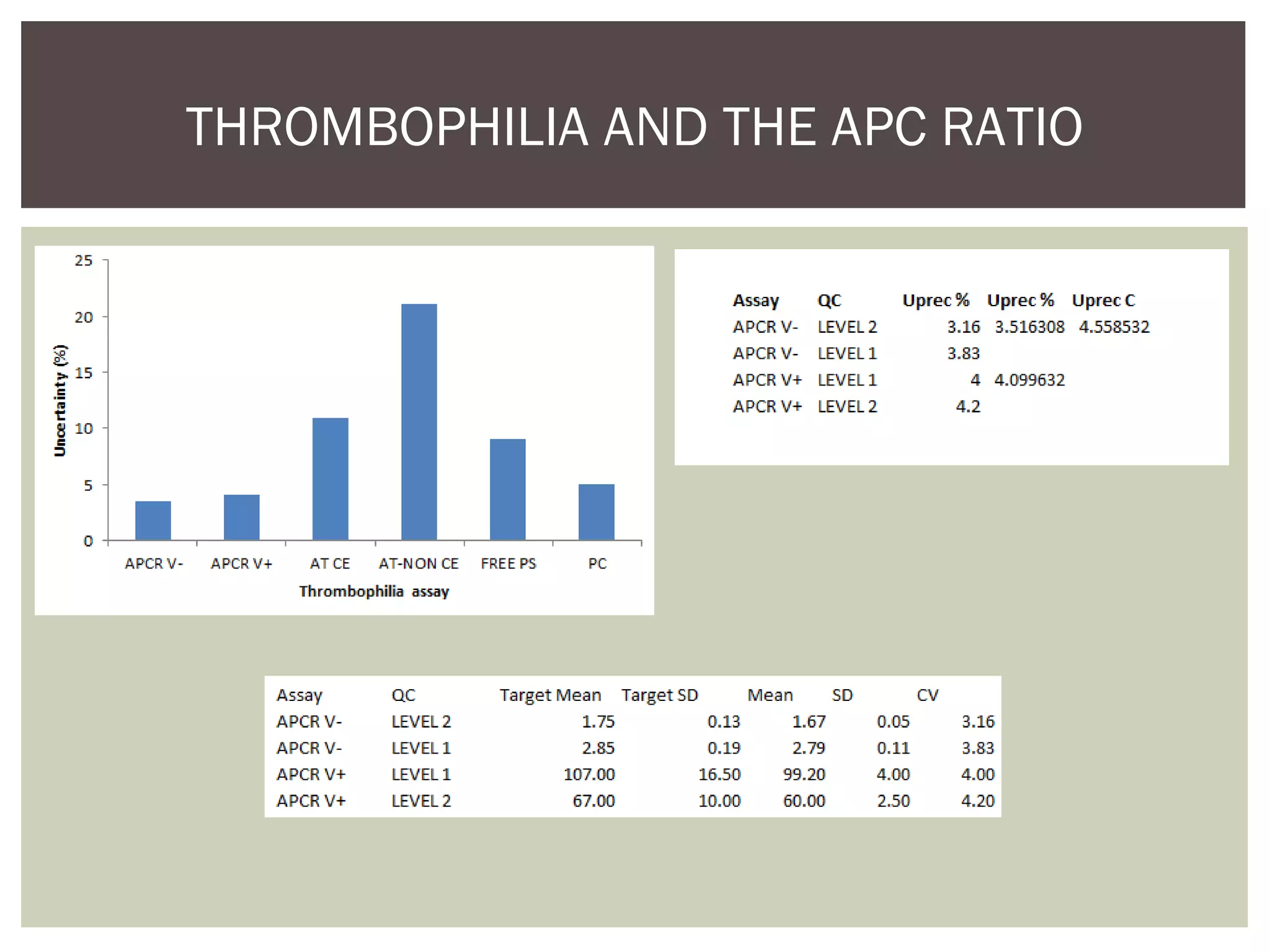
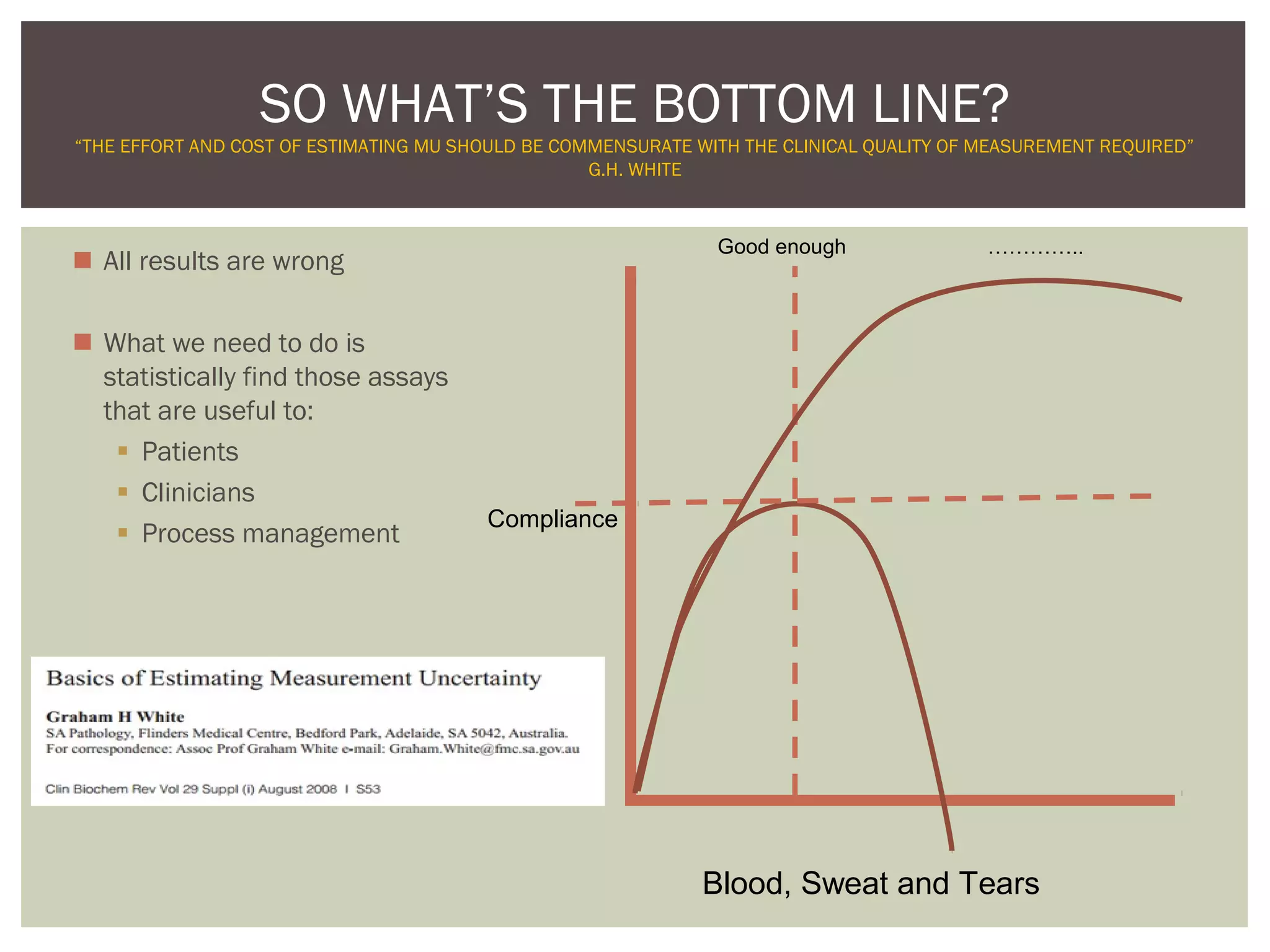
This document discusses uncertainty of measurement in haemostasis. It begins by outlining the speaker's disclosures and lack of conflicts of interest. It then discusses key concepts like accuracy vs precision, sources of error like bias and random error, and standards like ISO15189 that require estimating measurement uncertainty. The document outlines a seven-step process to account for sources of uncertainty, collect data, define the measurand, account for outliers, calculate imprecision, define significant figures, account for bias, and assess the data. Specific examples are provided for different types of haemostasis assays. The summary emphasizes estimating and reporting measurement uncertainty to provide useful information to patients, clinicians, and for process management.