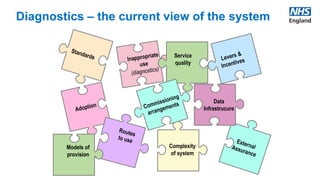
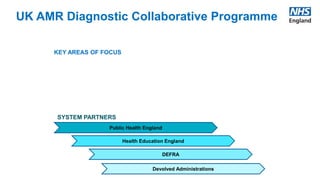
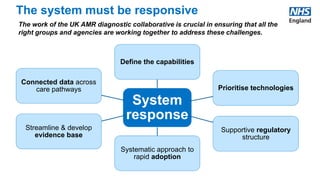
The UK AMR Diagnostic Collaborative aims to maximize the use of diagnostic technology to tackle antimicrobial resistance. It provides single point of focus and leadership for the complex UK diagnostic system. Key areas of focus include diagnostic stewardship, innovation, and addressing unwarranted variation. Upcoming milestones include a national blood culture survey and engagement with industry to accelerate innovative solutions. The collaborative works in partnership across agencies to ensure a responsive diagnostic system.