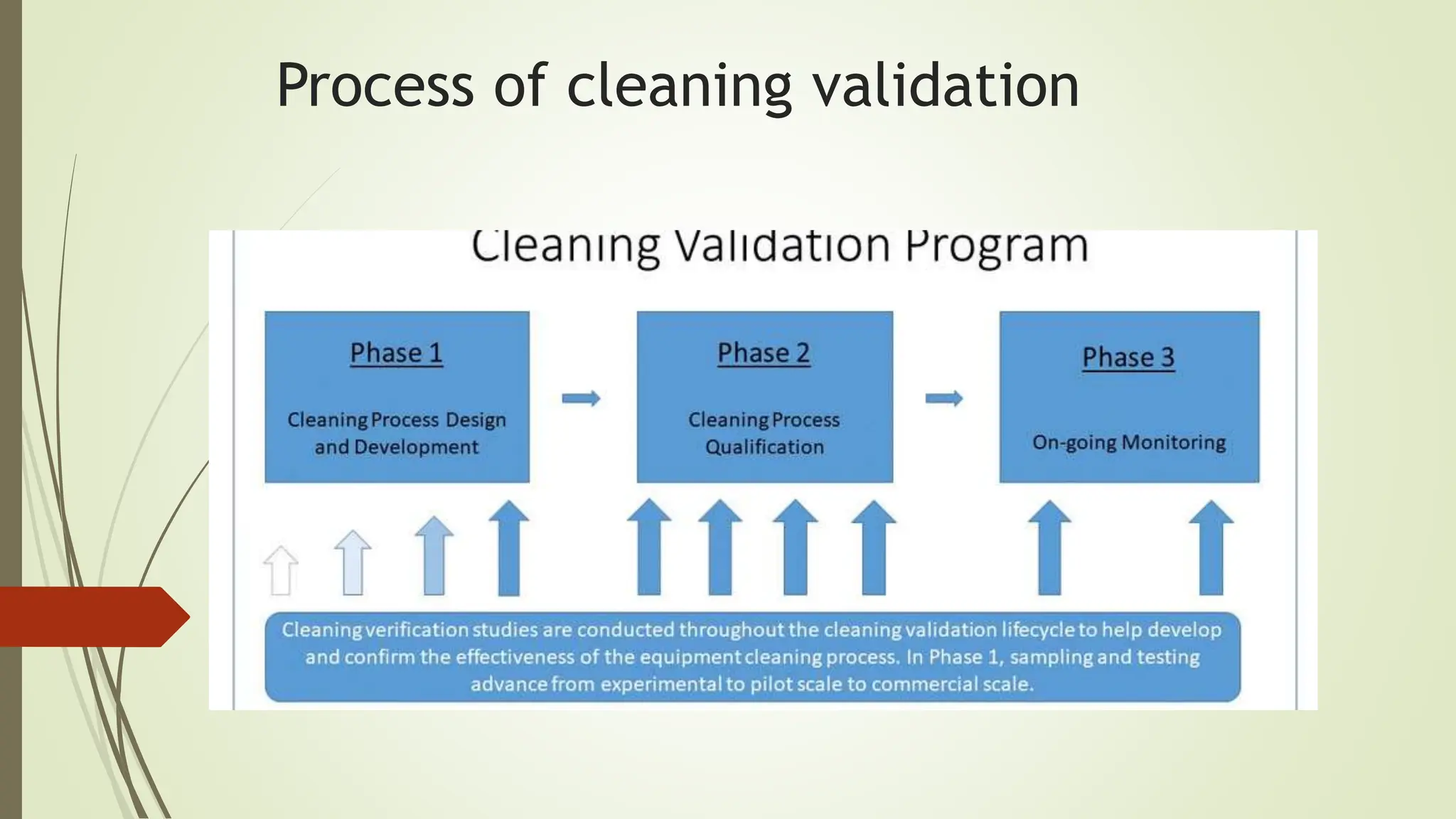
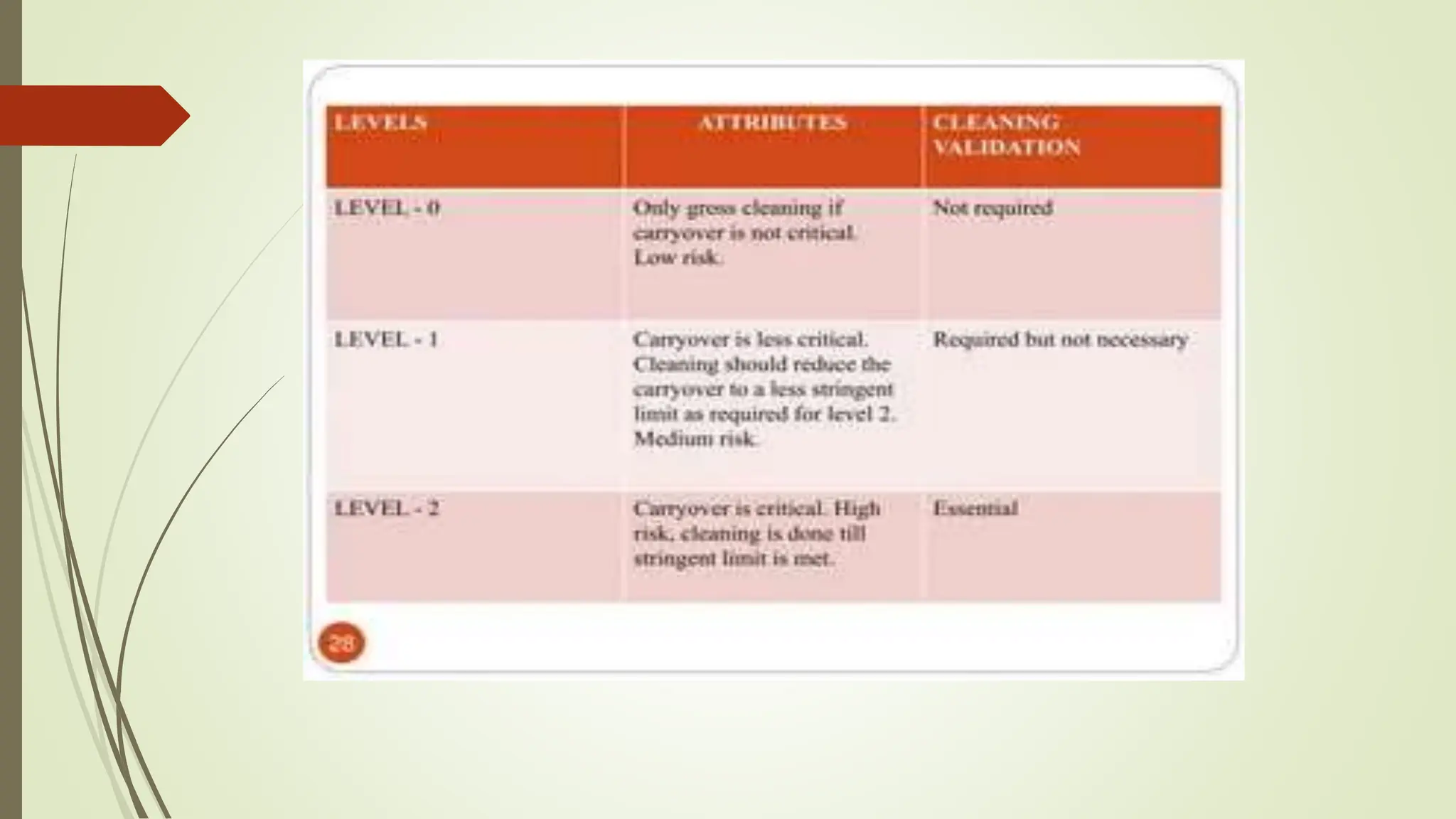
Cleaning validation is a methodology to ensure the removal of chemical and microbial residues from equipment to maintain product quality and prevent cross-contamination. A comprehensive cleaning validation protocol outlines objectives, sampling procedures, testing methods, and acceptance criteria. The overall cleaning process must adhere to safety and quality principles in pharmaceutical manufacturing.