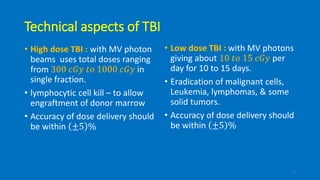
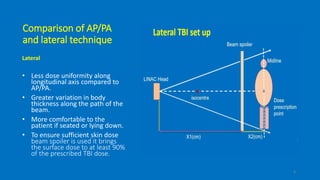
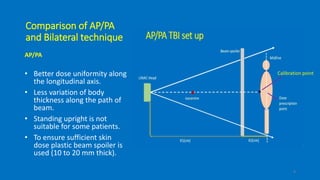
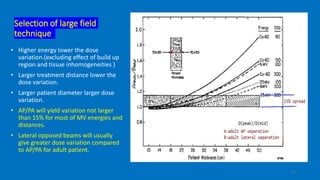
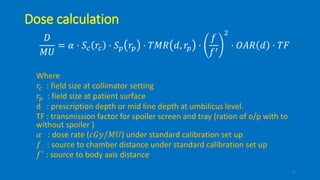
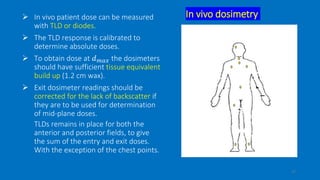
Total body irradiation (TBI) is a form of radiotherapy used prior to bone marrow transplants to reduce the risk of transplant rejection and destroy any remaining cancer cells. TBI techniques use large photon fields, usually from cobalt-60 machines or LINACs, to irradiate the entire body. Common techniques include opposing anterior-posterior beams or lateral beams. Precise dosimetry is required due to the large fields and total body exposure, with dose uniformity targets of within ±10% across the body. In vivo dosimetry using TLD or diodes is also employed to verify accurate dose delivery. Early side effects from TBI include fatigue, nausea, hair loss and skin irritation due to the whole body irradiation