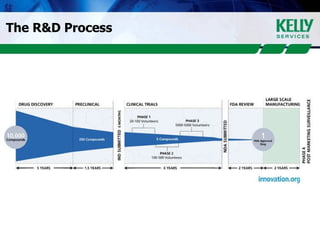
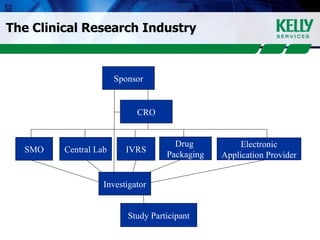
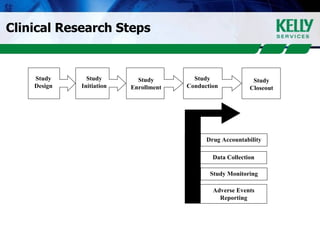
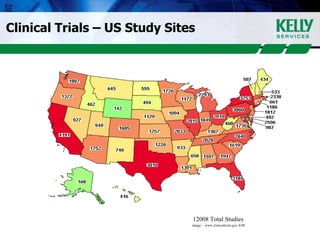
This document provides an overview of clinical research, including what clinical research is, the clinical research process, types of organizations involved, and the steps involved in clinical trials. It then discusses KSR Clinical Research, a consulting firm that places clinical research professionals with pharmaceutical, biotech, and medical device companies as well as research facilities. KSR provides recruitment, project management support, and competencies across various clinical research functions and therapeutic areas.