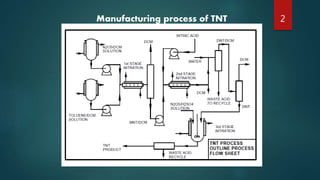
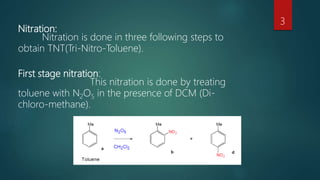
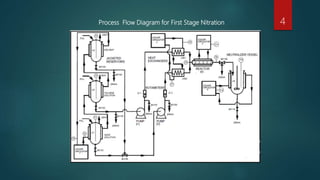
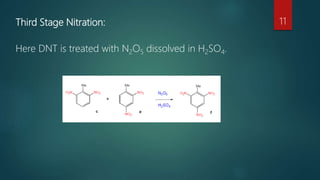
The document describes the manufacturing process for TNT (tri-nitro-toluene) through a three stage nitration process. The process involves:
1) Nitrating toluene with nitrogen pentoxide in the presence of dichloromethane to produce mono-nitro-toluene.
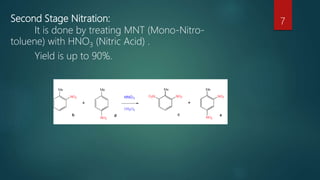
2) Treating the mono-nitro-toluene with nitric acid to produce di-nitro-toluene.
3) Reacting the di-nitro-toluene with nitrogen pentoxide dissolved in sulfuric acid to produce TNT.
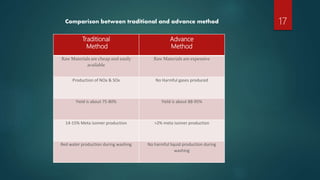
The process aims to control temperatures, residence times, and yields at each stage to minimize unwanted meta-isomer byproducts and maximize