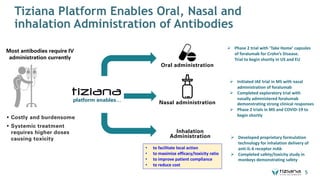
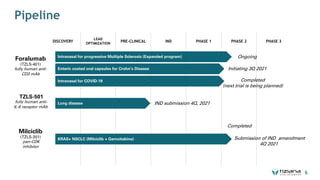
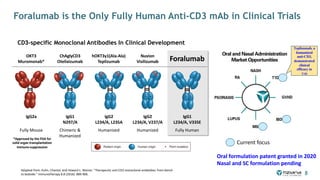
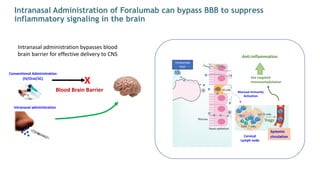
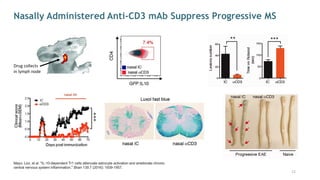
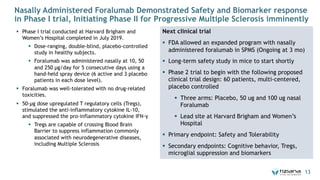
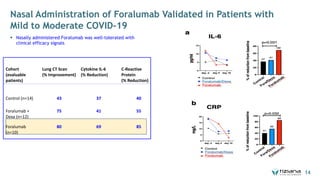
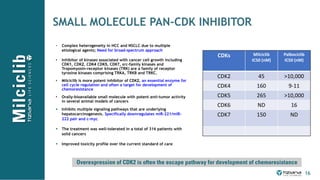
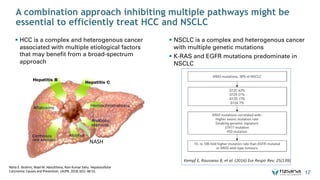
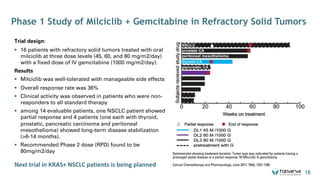
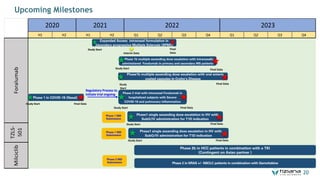
The document is a corporate presentation for Tiziana Life Sciences, outlining its business and forward-looking statements regarding clinical trials and drug developments. It highlights the management team's expertise and the company's innovative platforms for administering antibodies, including oral and nasal methods. The presentation includes information on various drugs in the pipeline, particularly for the treatment of Crohn's disease, progressive multiple sclerosis, and non-small cell lung cancer.