Genetic Technologies is an Australian company focused on developing and commercializing genetic risk assessment tests. The presentation provides an overview of the company's business, including its portfolio of current and upcoming genetic tests, markets and channels to market, and capabilities. Key points include:
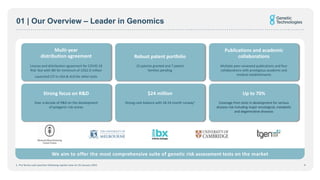
- Genetic Technologies aims to offer the most comprehensive suite of genetic risk assessment tests on the market, covering up to 70% of disease risks.
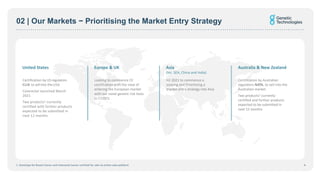
- The company is prioritizing market entry strategies in key regions like Australia, the US, and Asia.
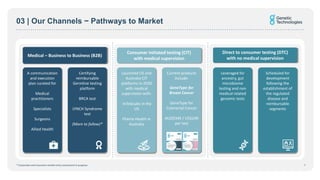
- Tests are marketed through consumer-initiated and medical business-to-business channels.
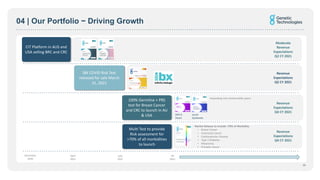
- An innovative pipeline of new tests for diseases like cancer, heart disease, and diabetes is being