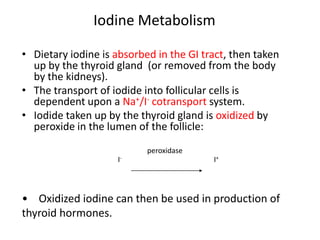
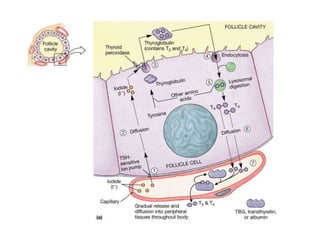
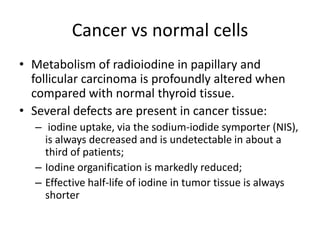
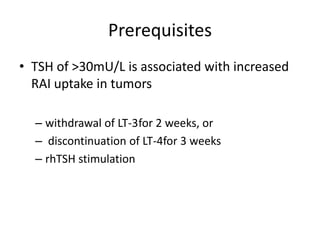
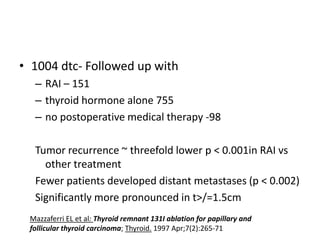
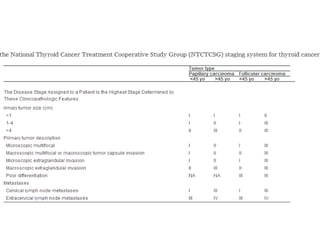
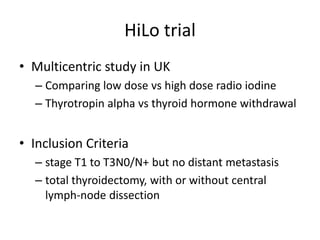
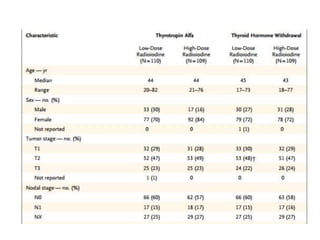
This document discusses the use of radioactive iodine (131I) for diagnosis and treatment of thyroid cancer. Some key points:
- 131I localizes in thyroid tissue and can be used to ablate thyroid remnants after surgery or treat thyroid cancer metastases. It emits beta and gamma radiation.
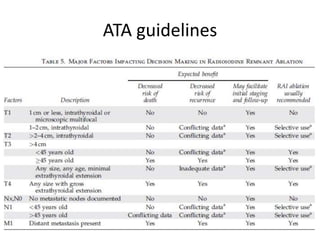
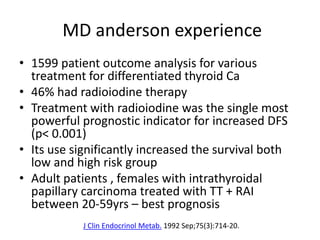
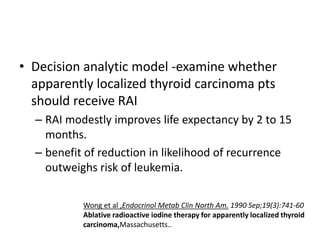
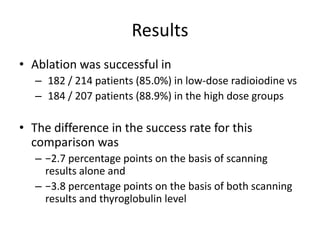
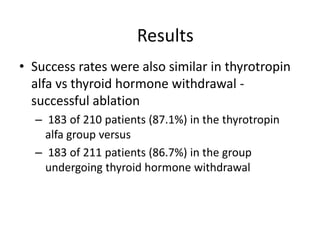
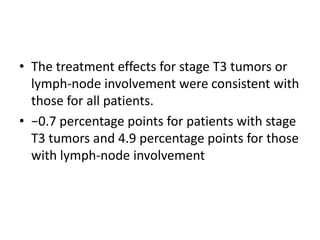
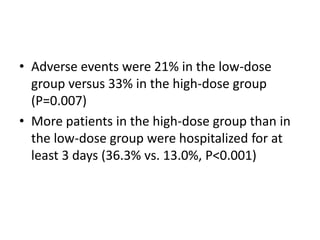
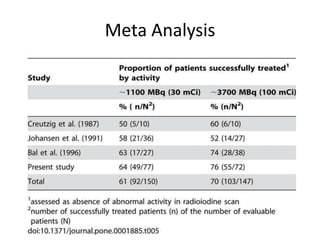
- For remnant ablation, lower doses (30-100 mCi) are usually sufficient while higher doses (100-200 mCi) may be needed for more aggressive cancers. Success rates are similar between low vs high doses and thyroid hormone withdrawal vs rhTSH.
- Post-therapy scans 2-10 days after treatment can identify additional metastases not seen on diagnostic scans in 10-26% of