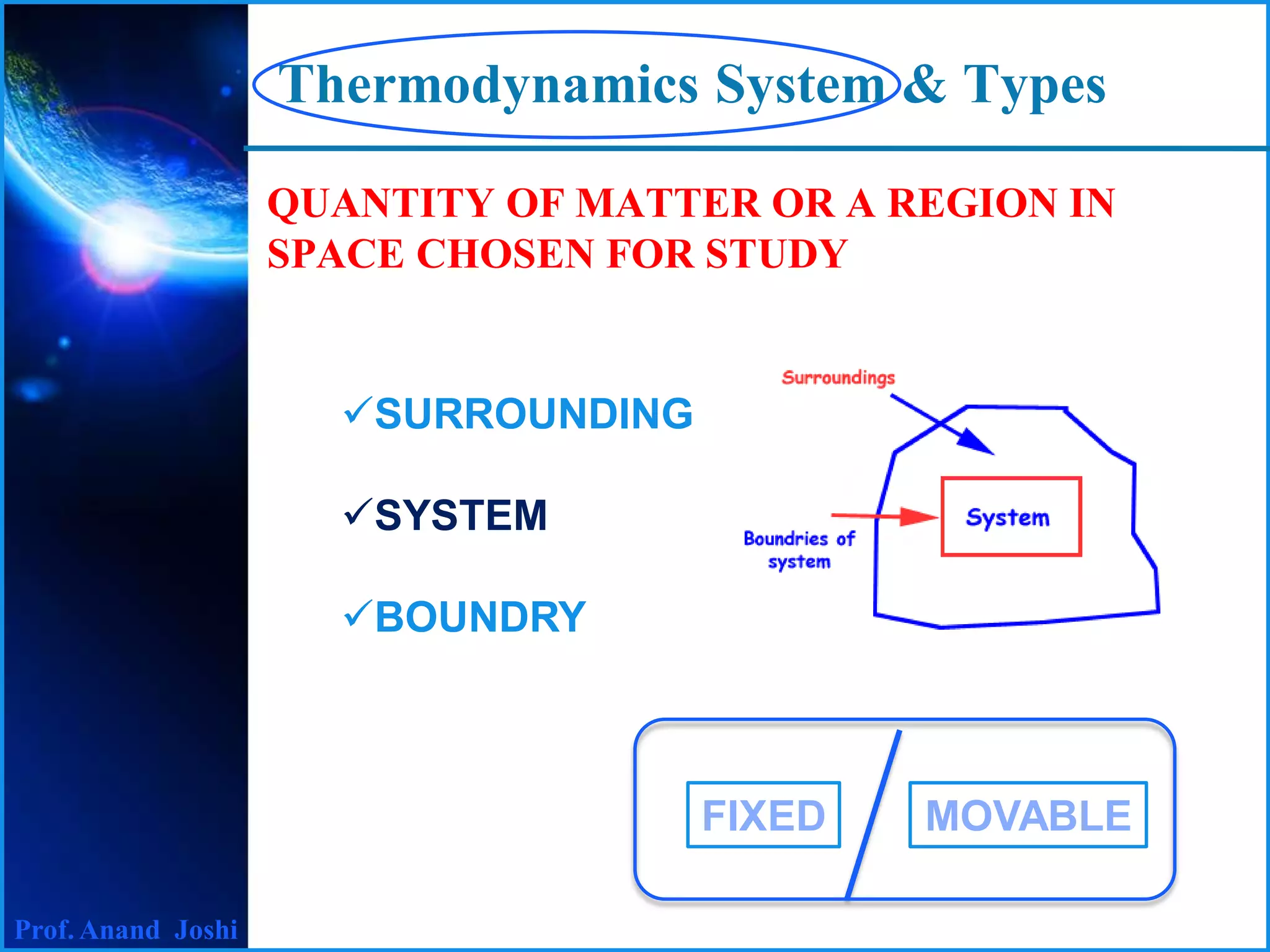
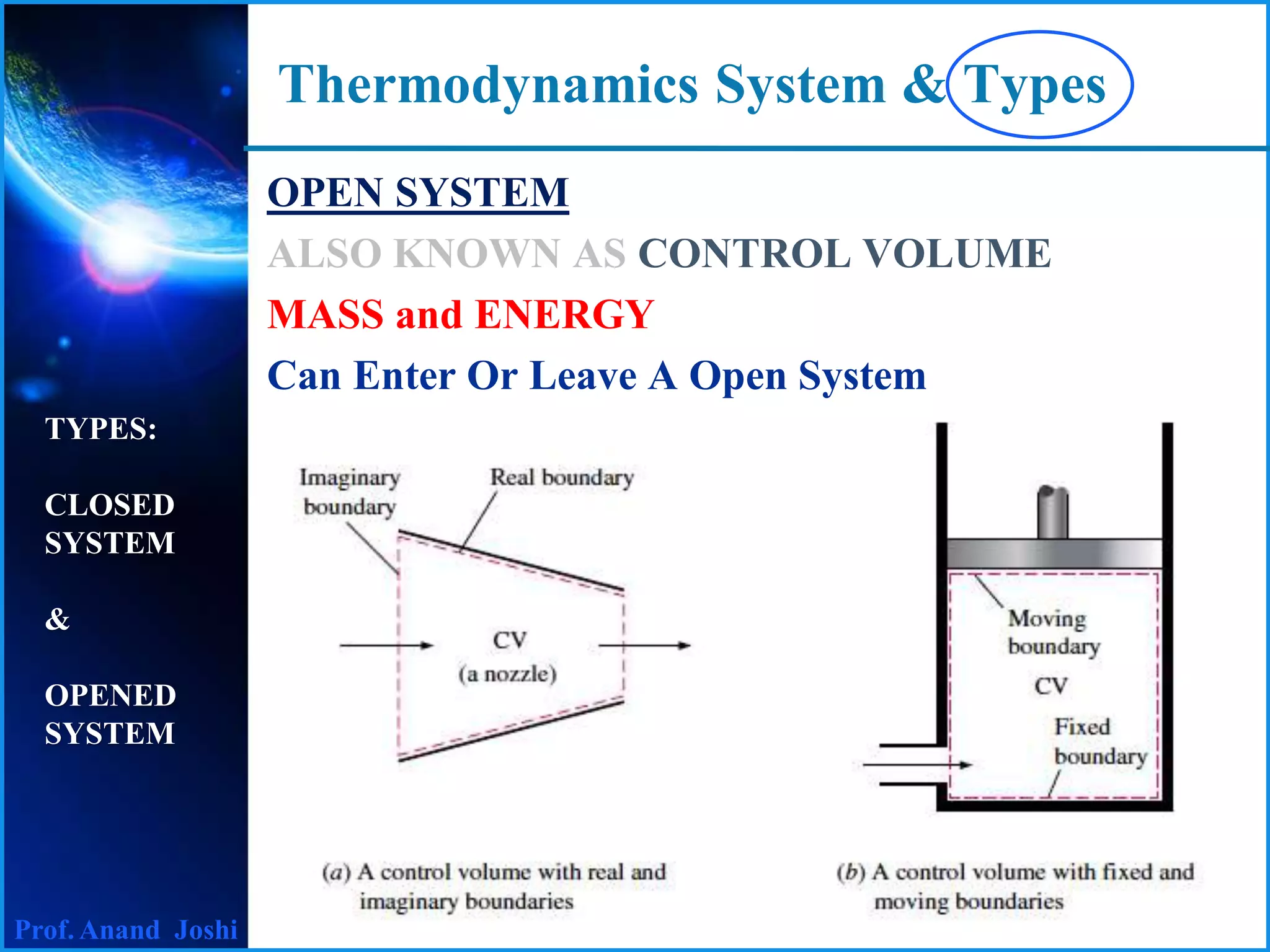
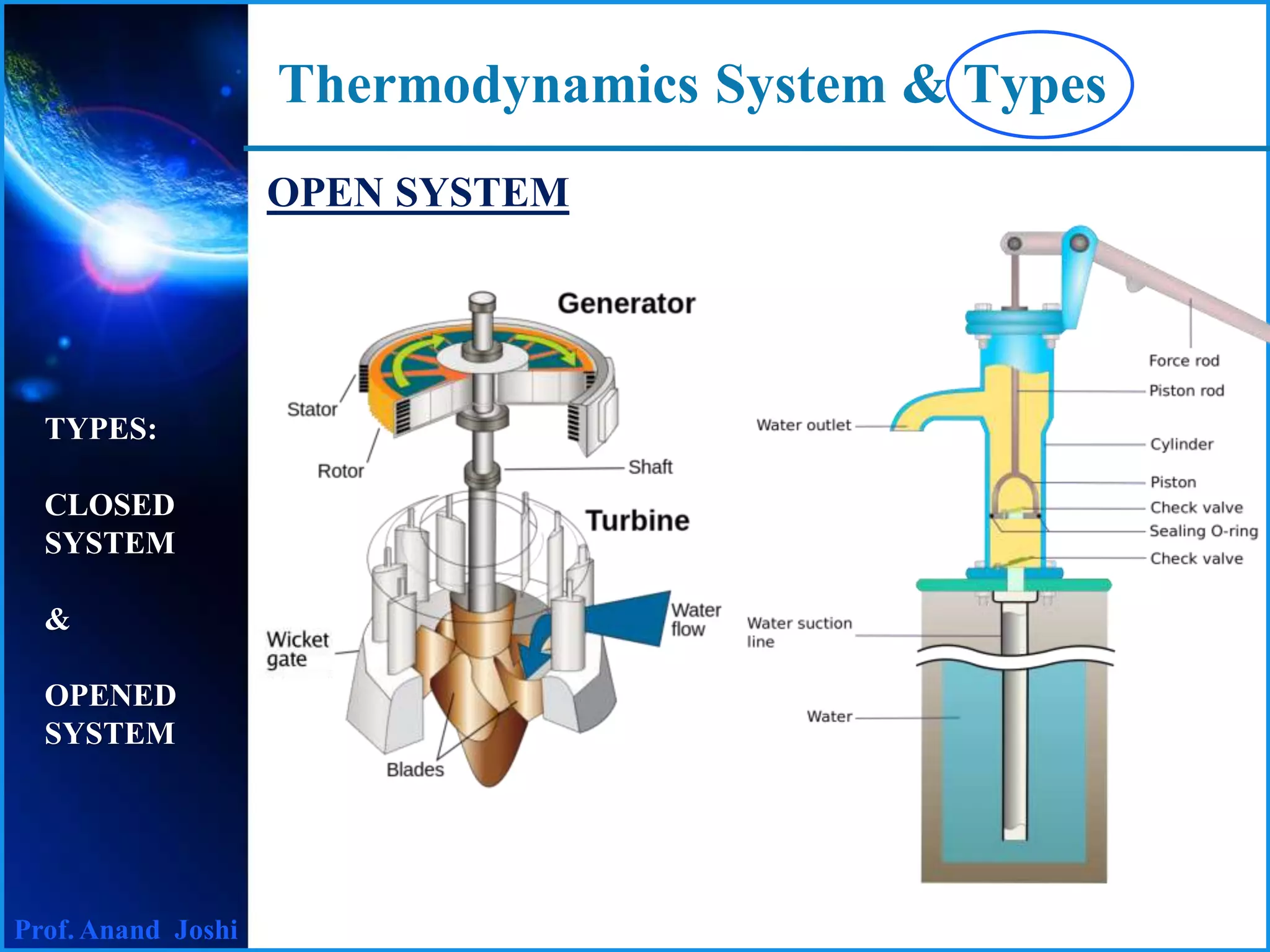
This document discusses thermodynamics systems and types. It defines a system, surroundings, and boundary, noting that the boundary is imaginary with zero thickness. It describes closed and open systems. A closed system is also called a control mass, where mass cannot enter or leave but energy in the form of heat or work can cross the boundary. An isolated system is a closed system that also does not allow heat transfer. An open system, also called a control volume, allows both mass and energy to enter or leave. Examples of closed, isolated, and open systems are provided.