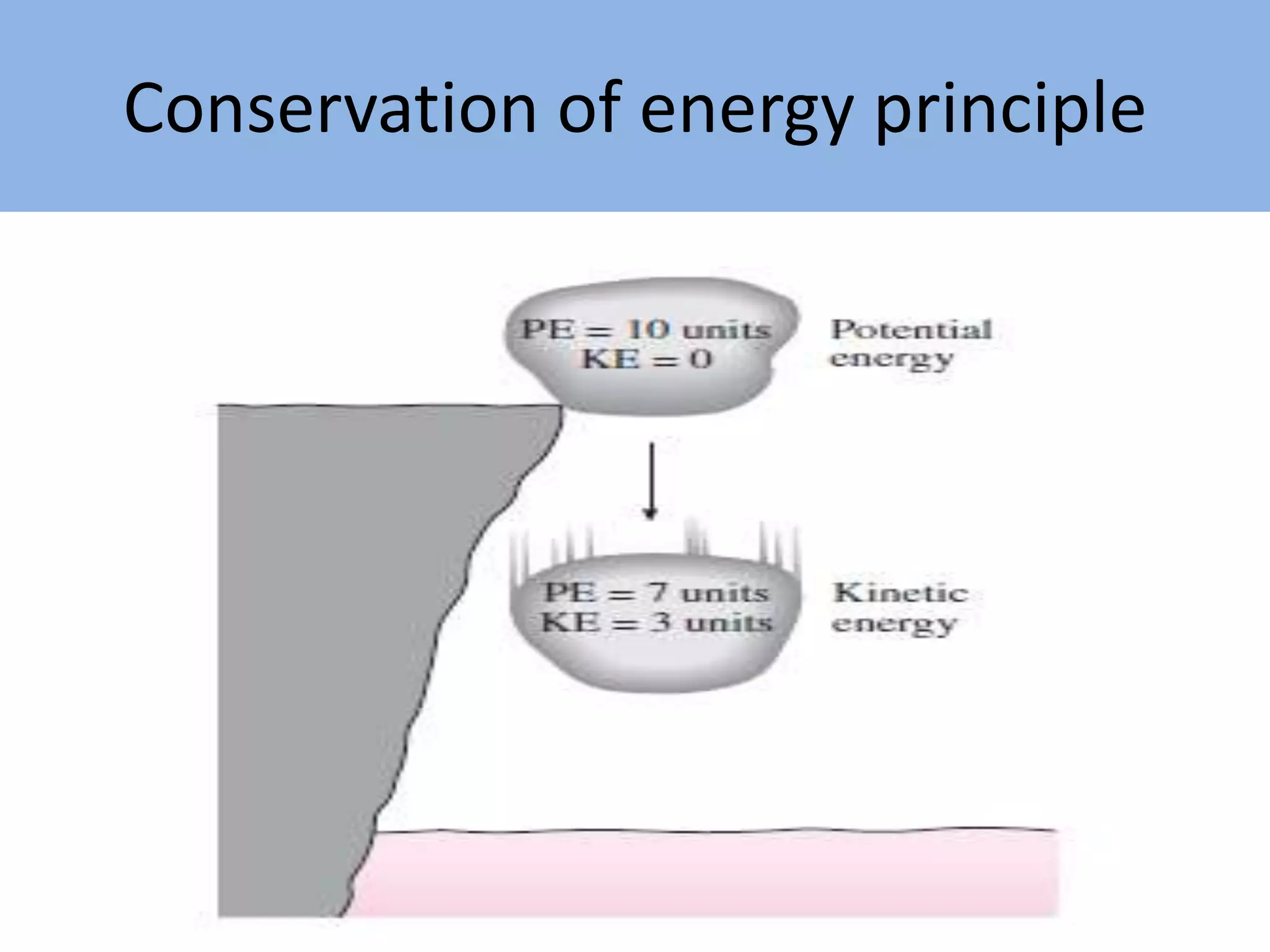
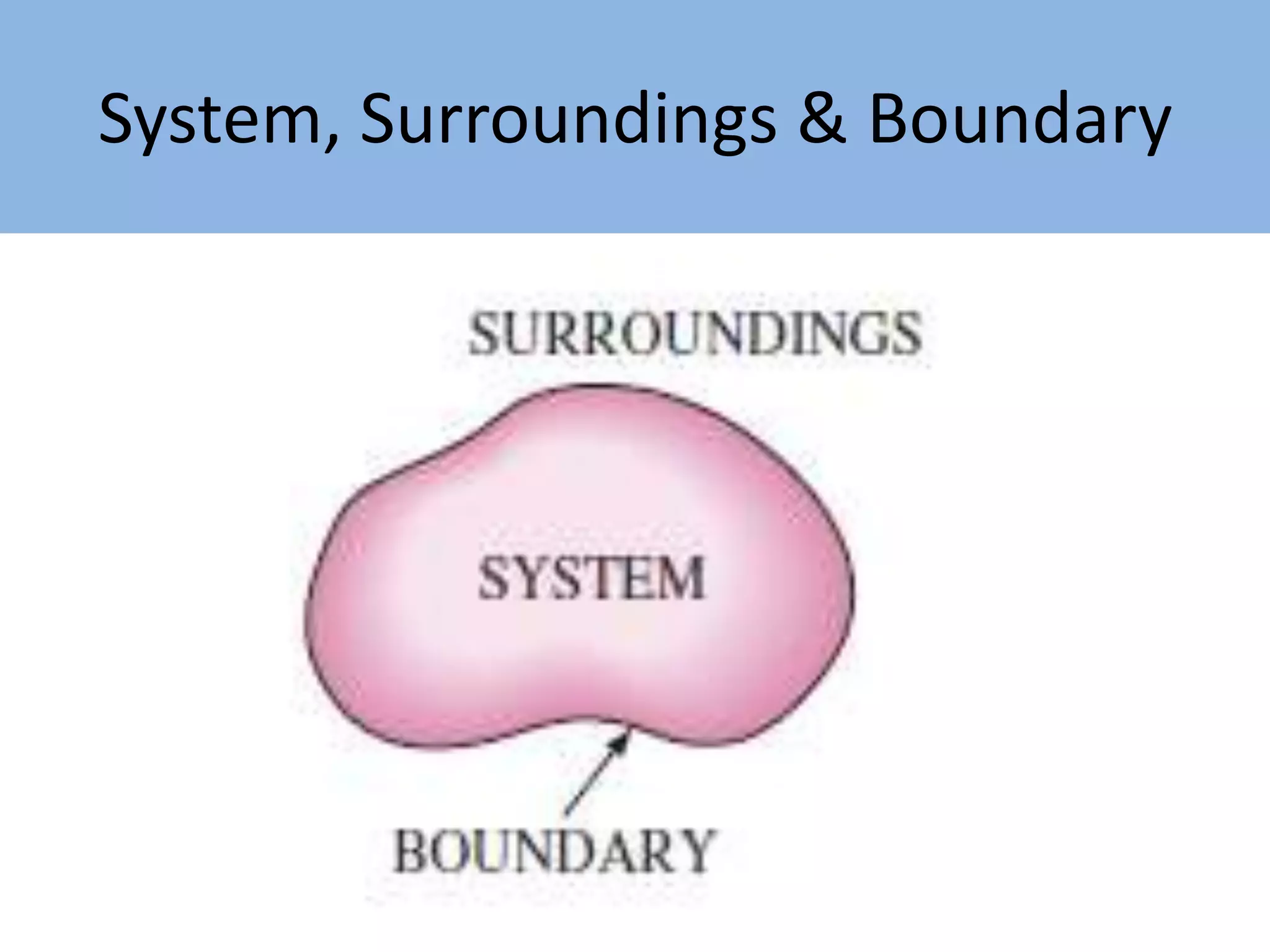
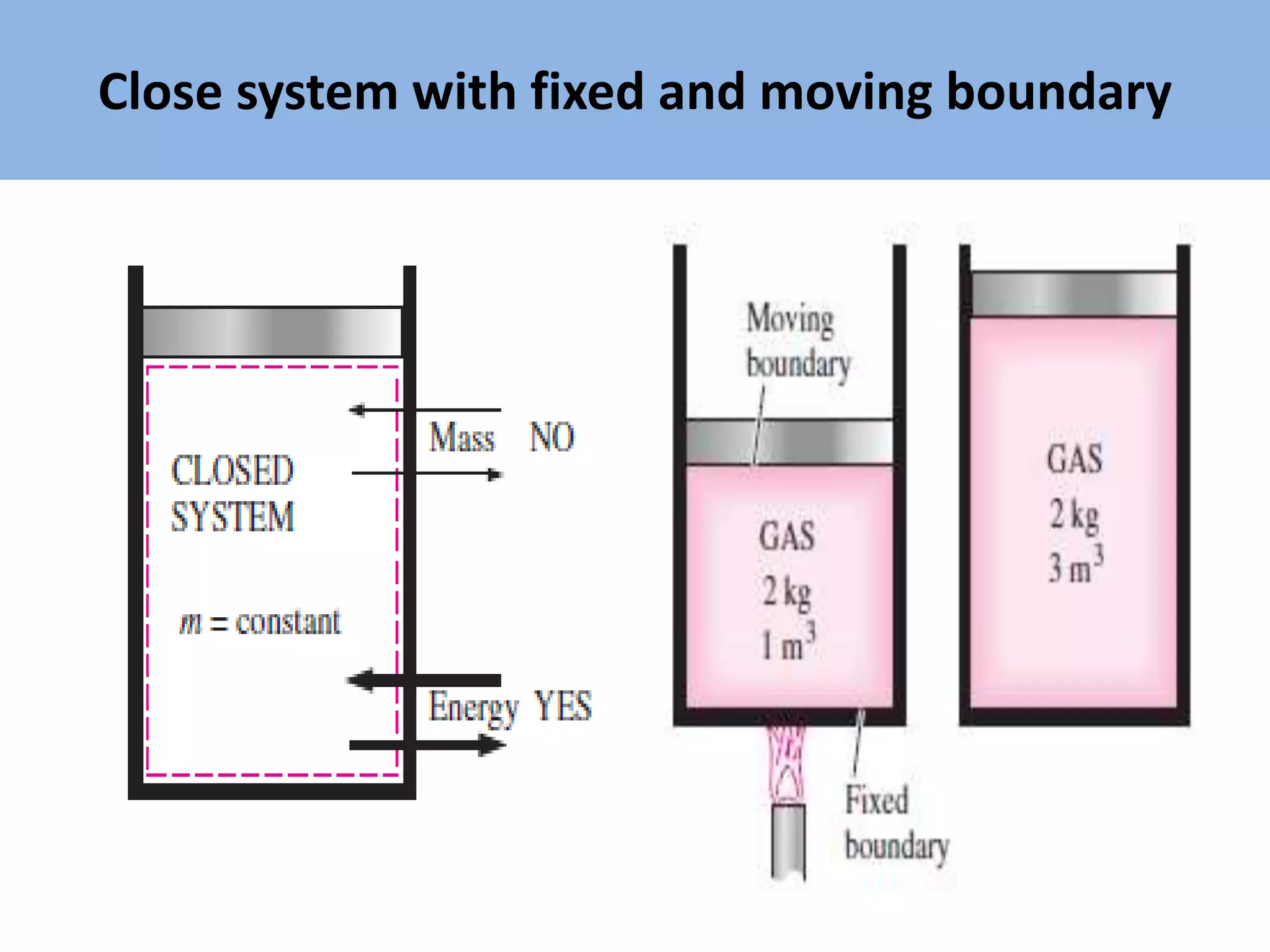
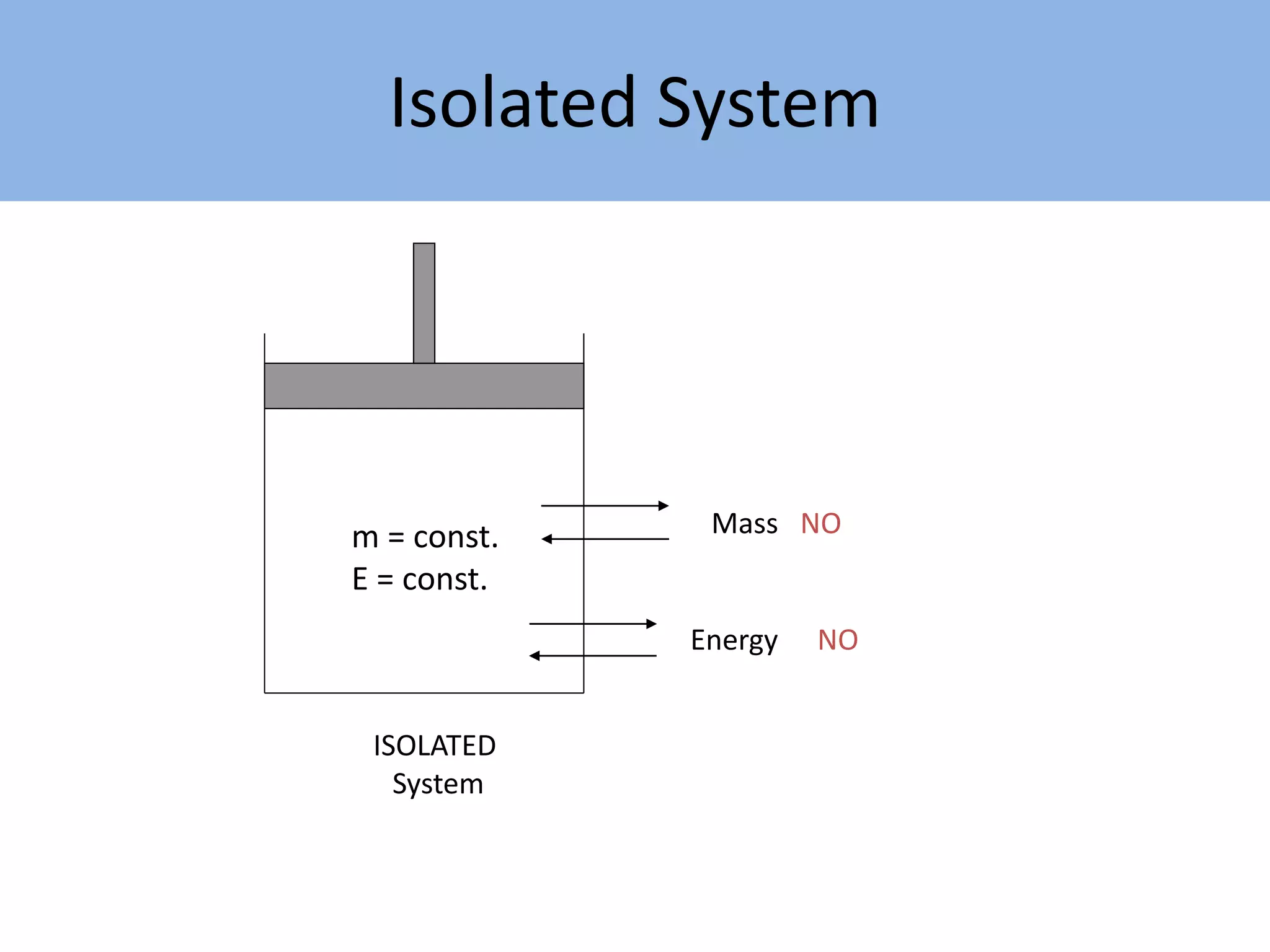
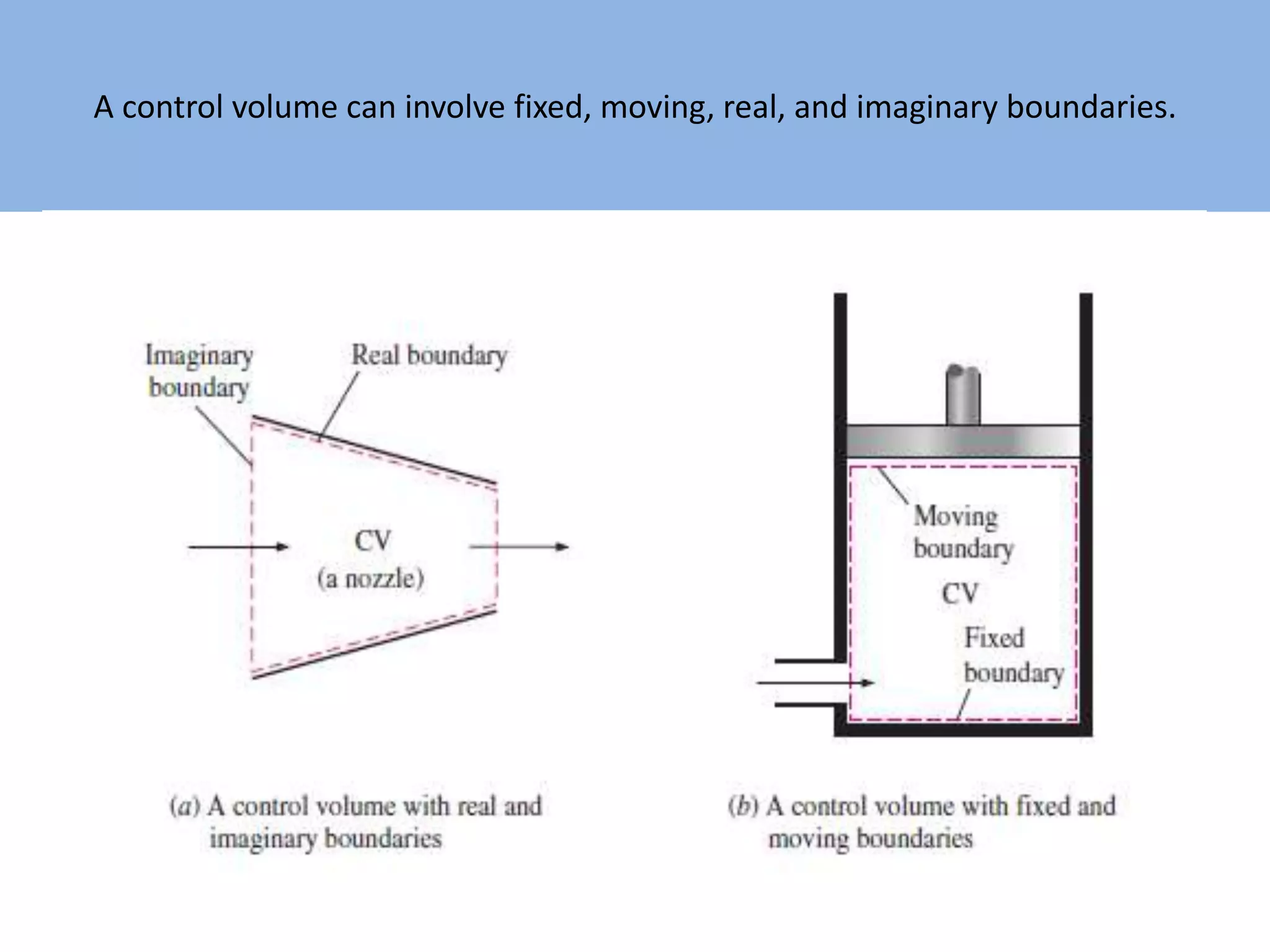
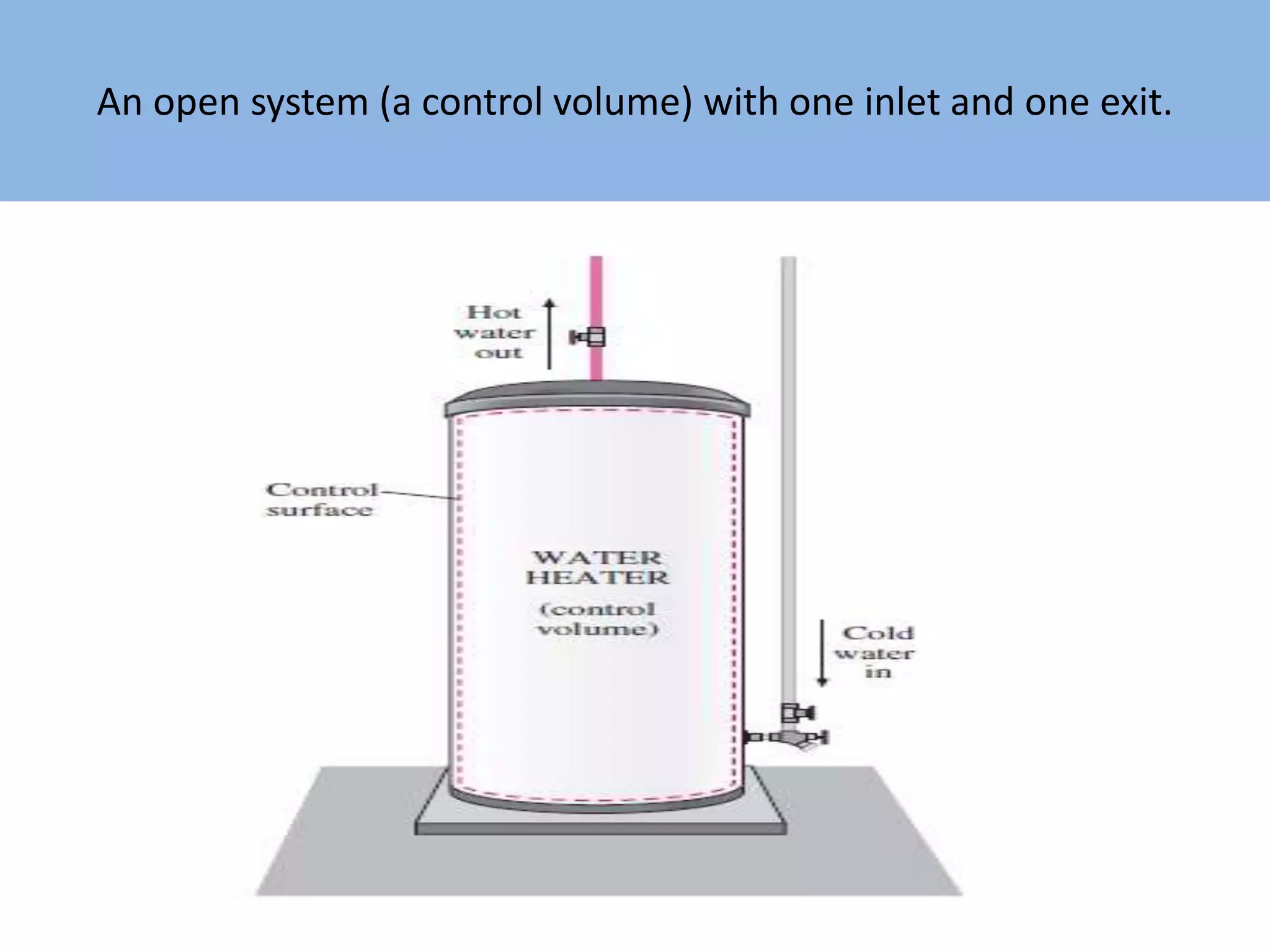
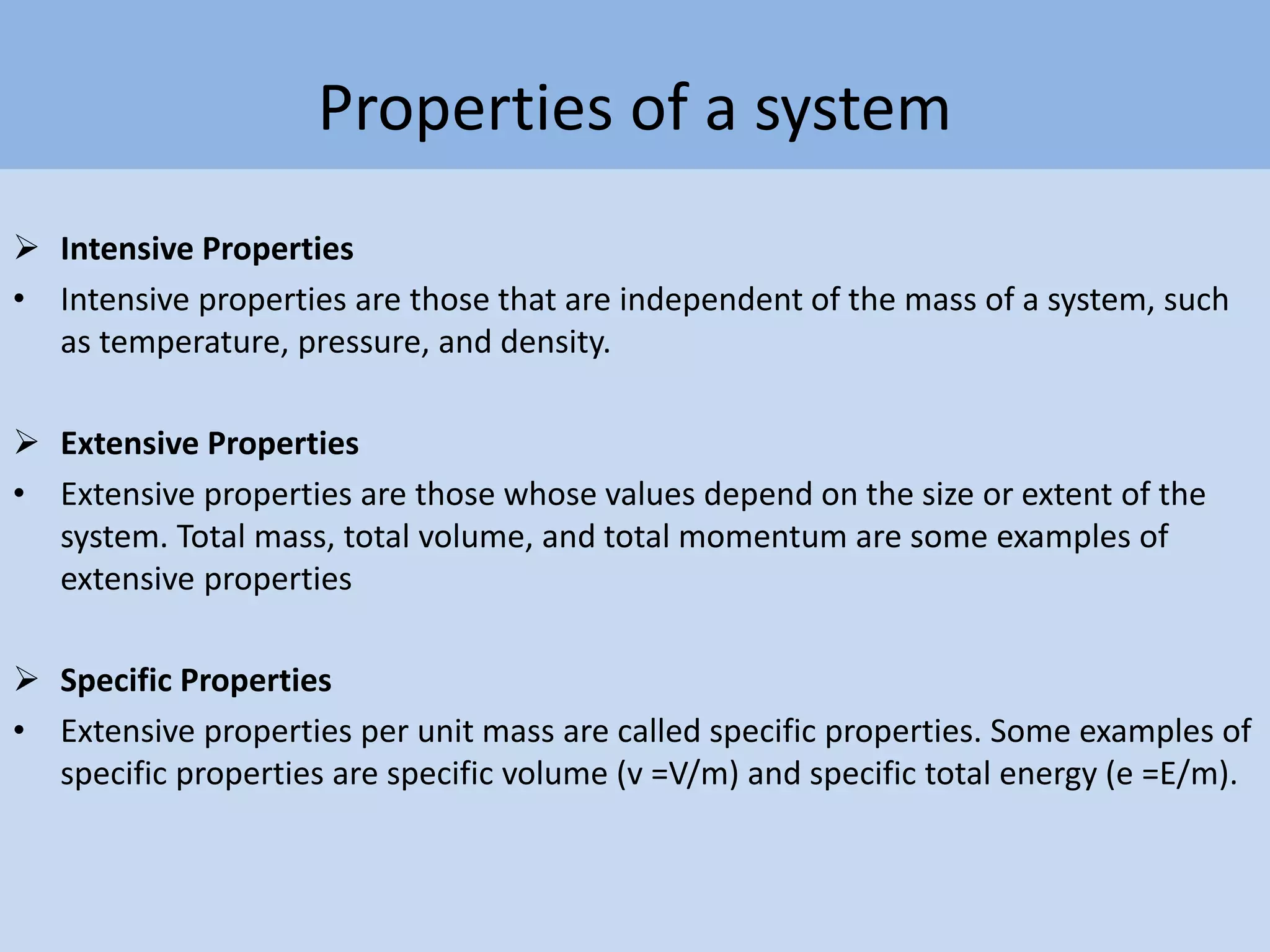
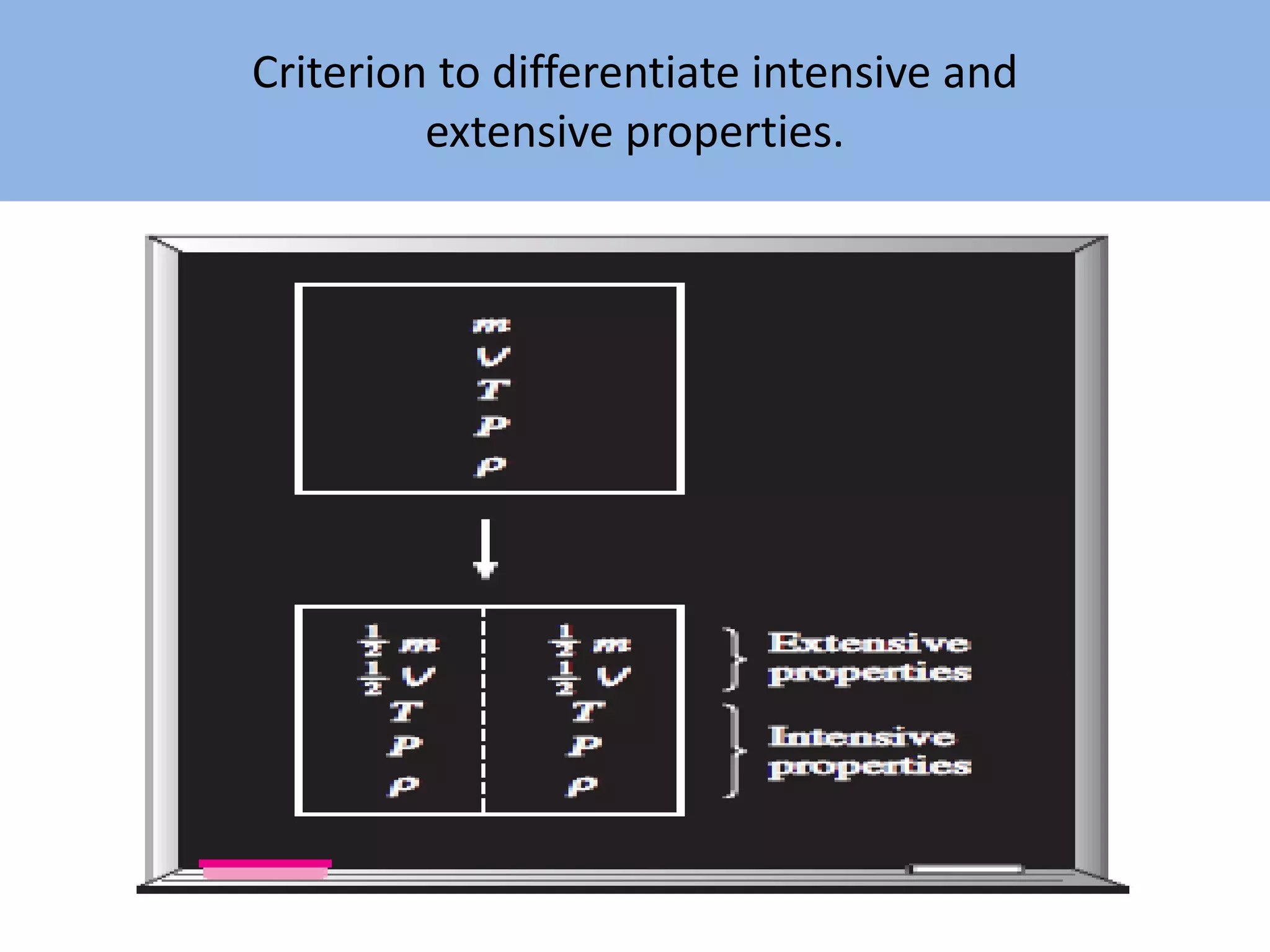
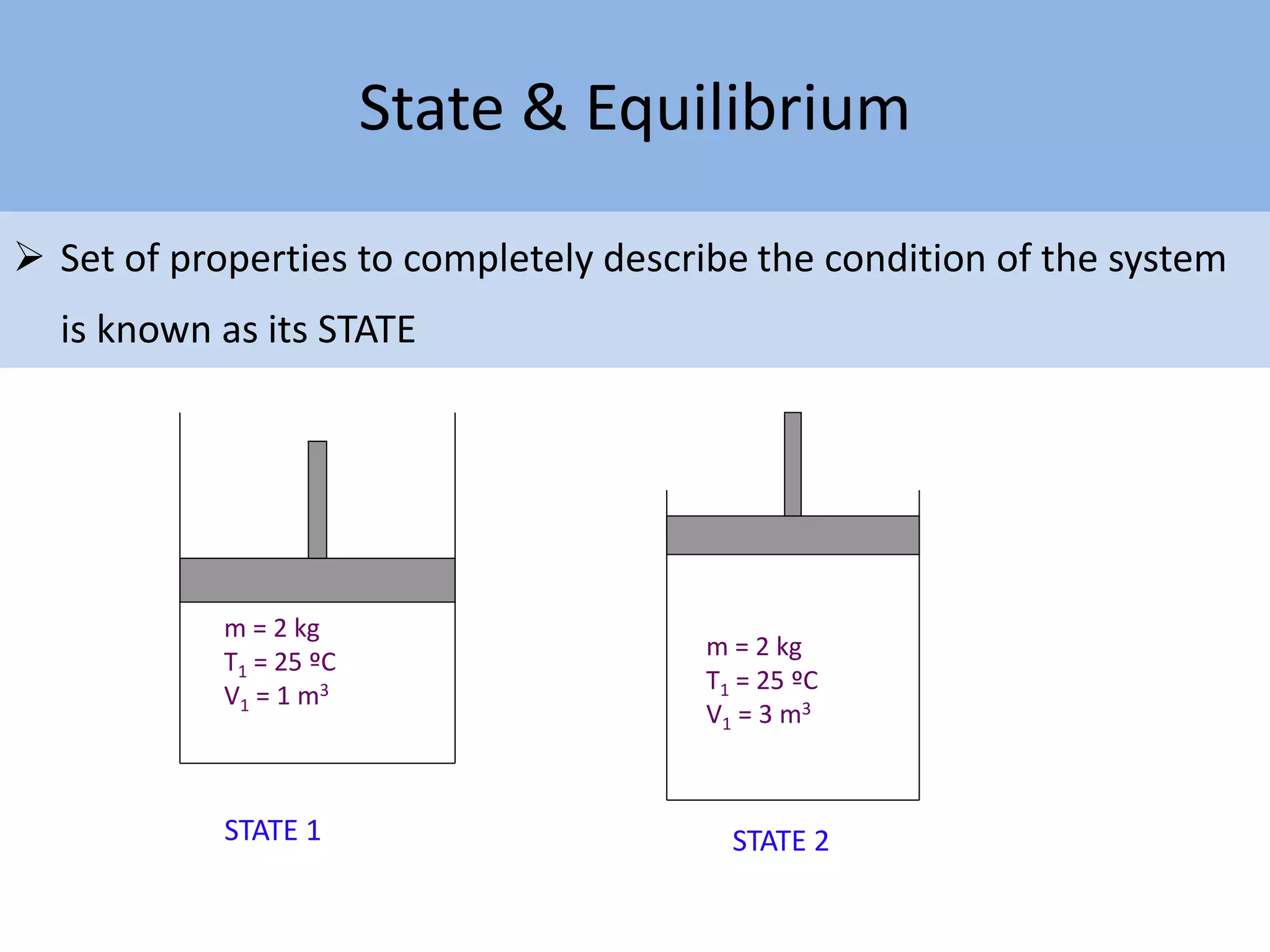
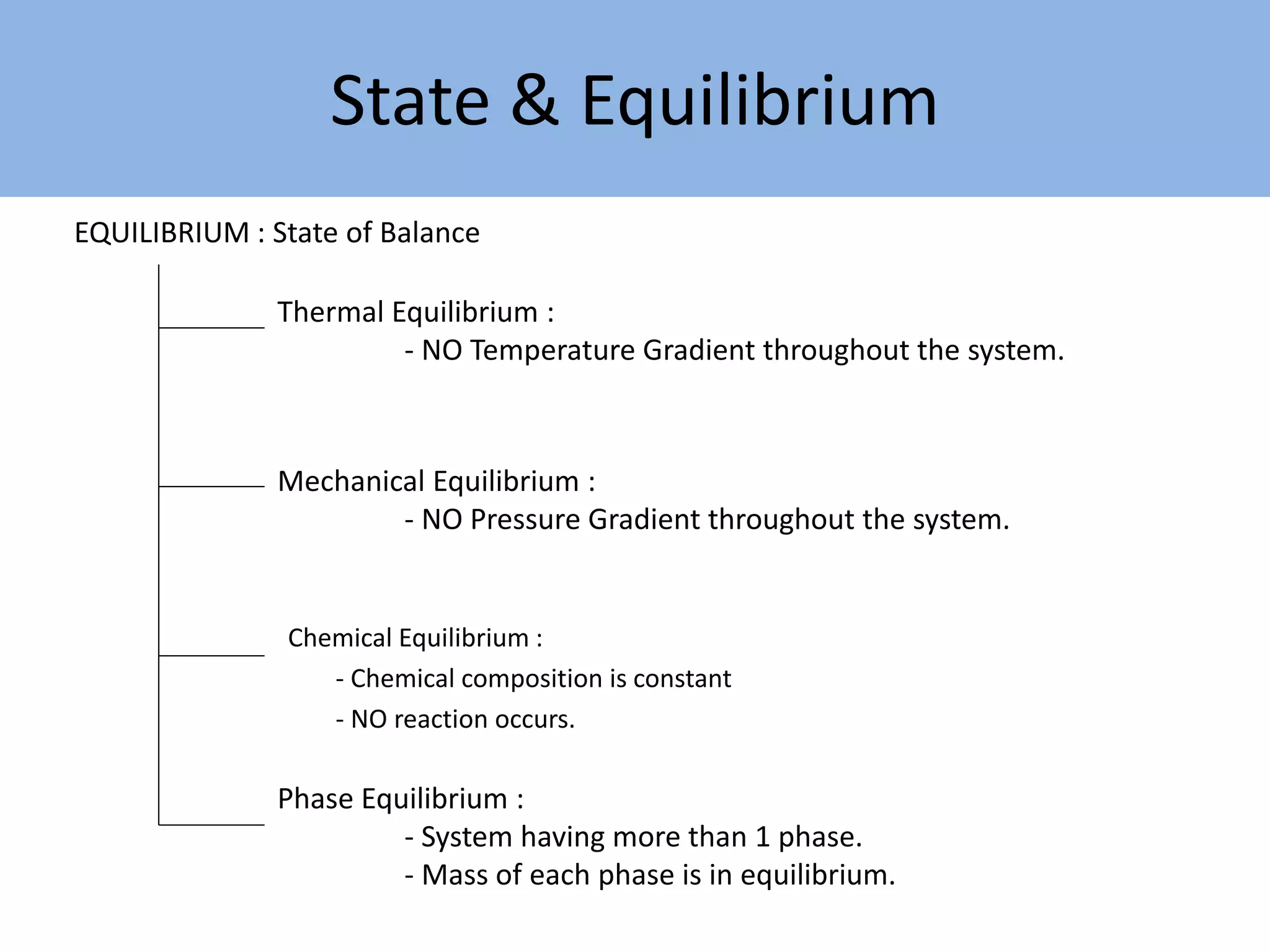
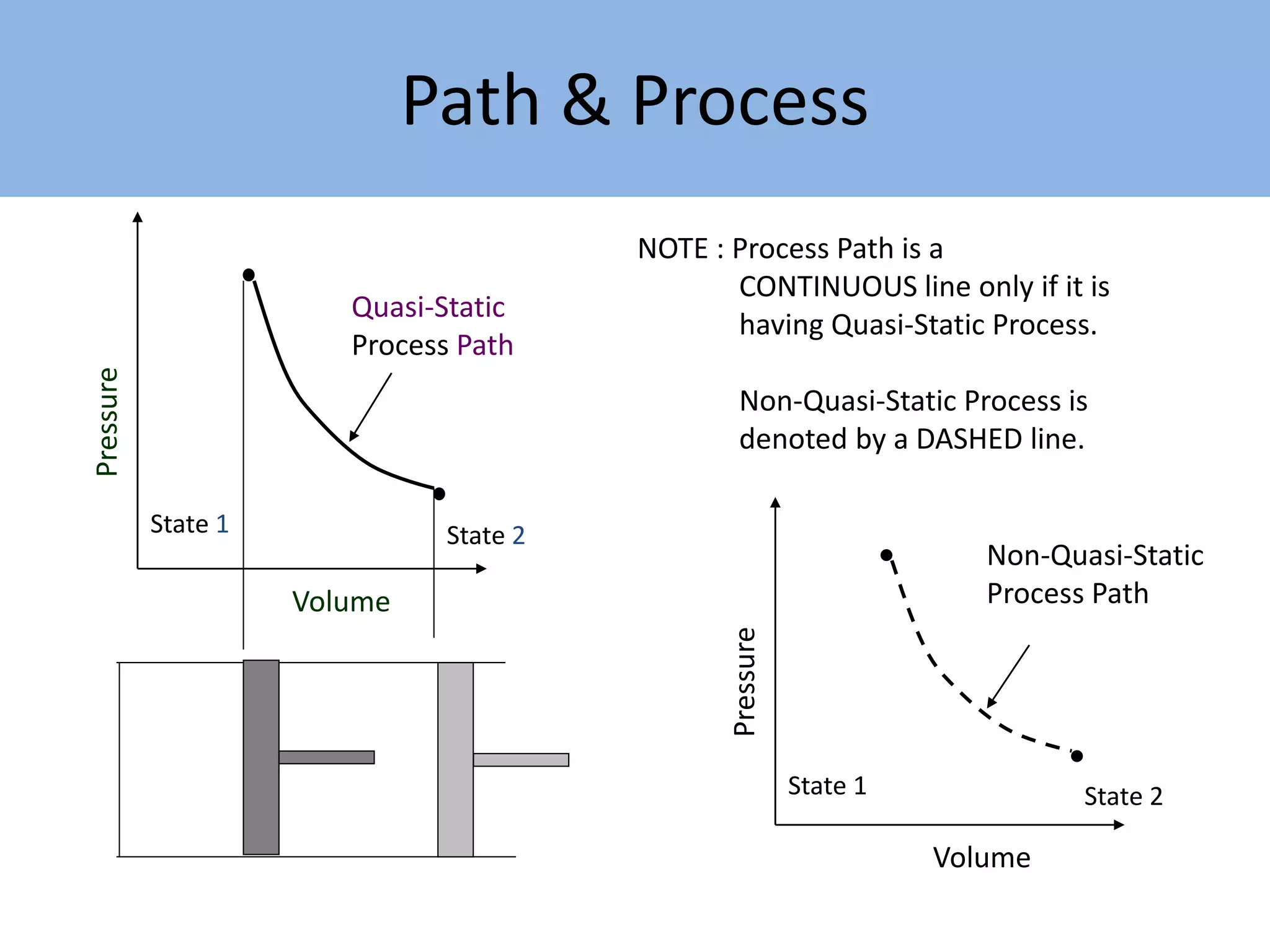
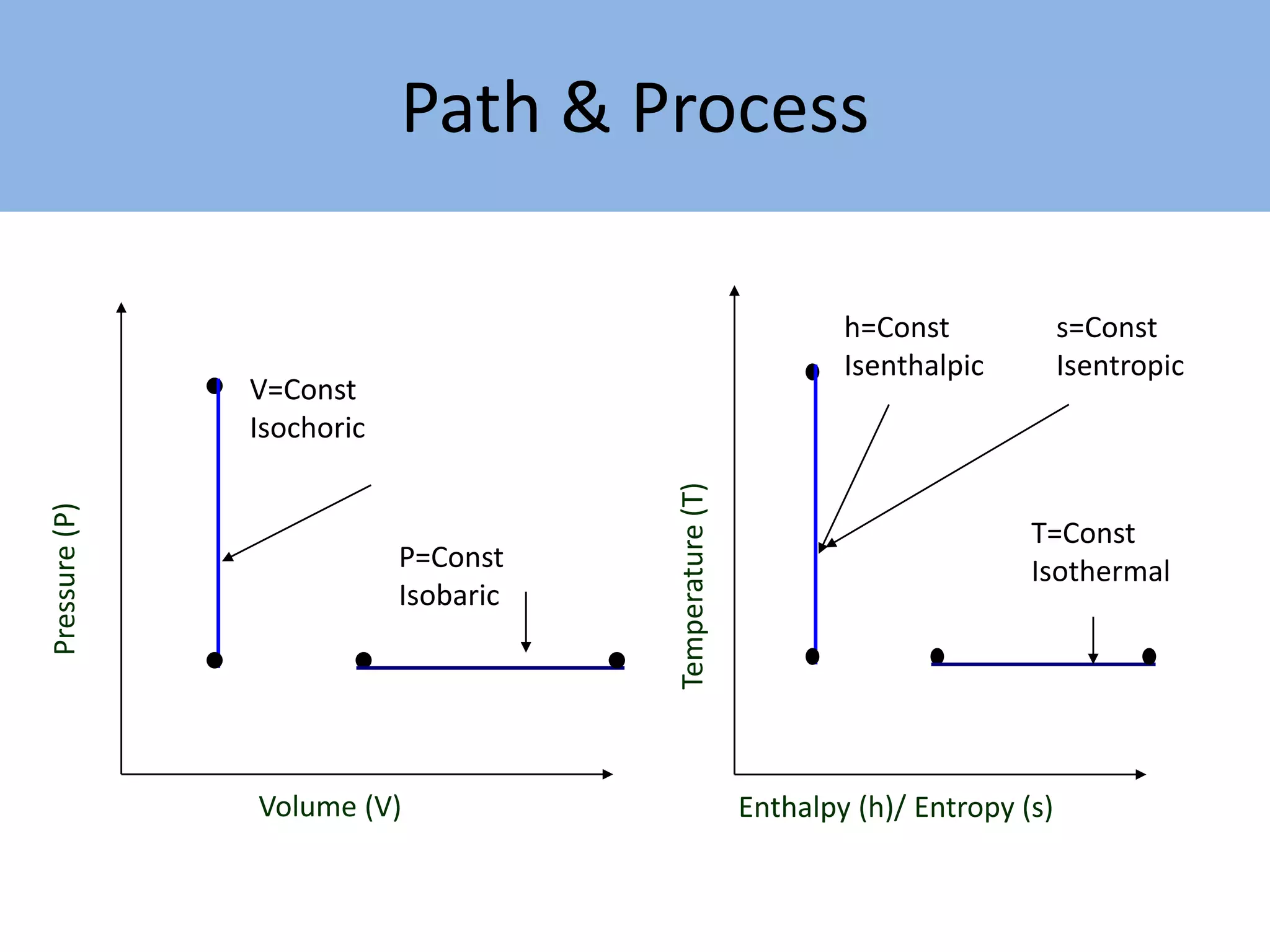
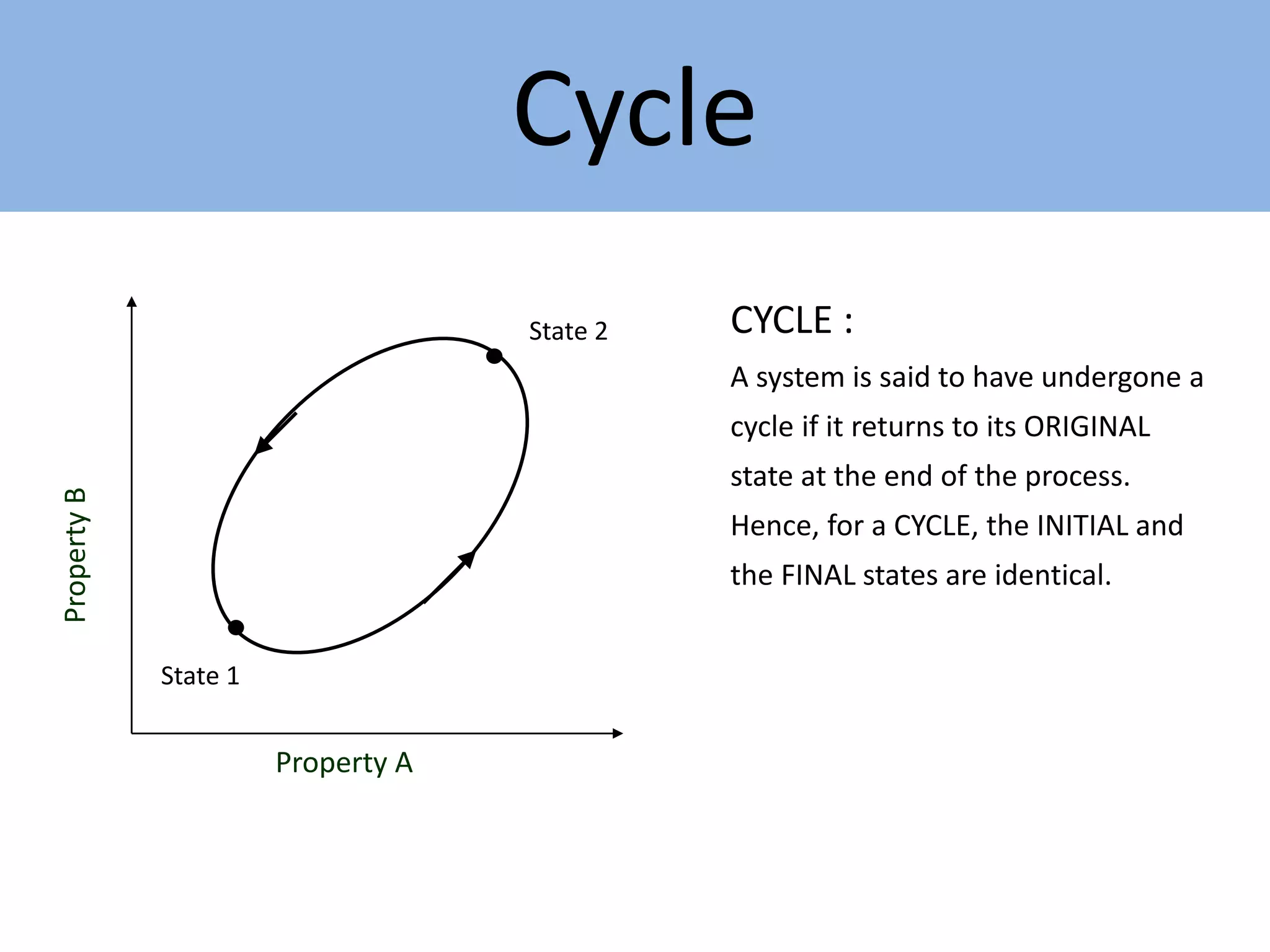
Thermodynamics is defined as the science of energy. It studies the transformation of heat into mechanical work and vice versa. Thermodynamics has applications in systems like the human body, refrigerators, engines, turbines, heaters, and solar collectors. A system is defined as the quantity of matter under study, surrounded by its surroundings. A boundary separates the system and surroundings. Closed systems do not allow mass transfer while open systems do. Equilibrium exists when properties do not vary within a system. State refers to the condition defined by properties like temperature, pressure and volume. Quasi-static processes are reversible while non-quasi-static processes are irreversible. Cycles occur when a system returns to its original state.