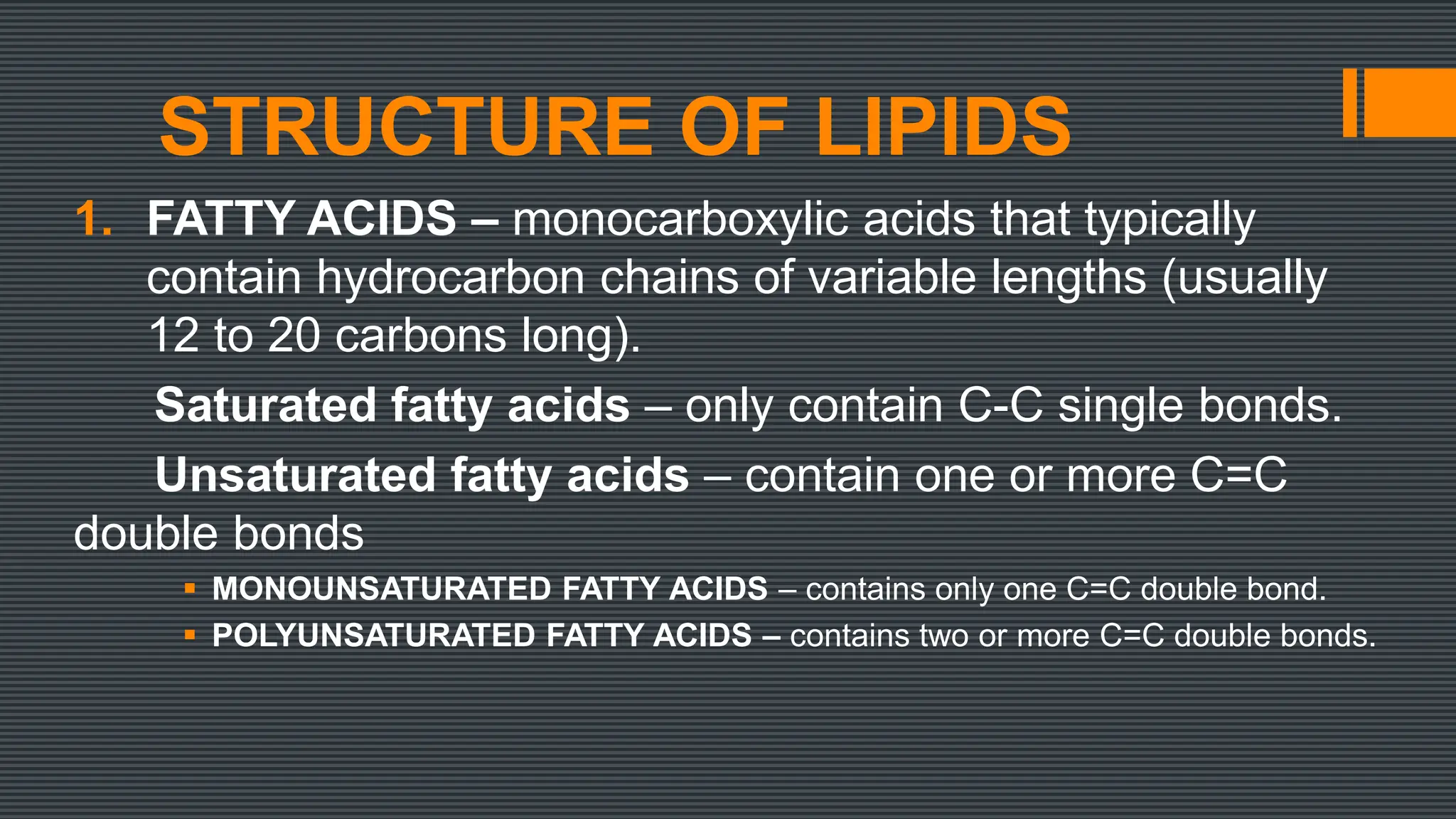
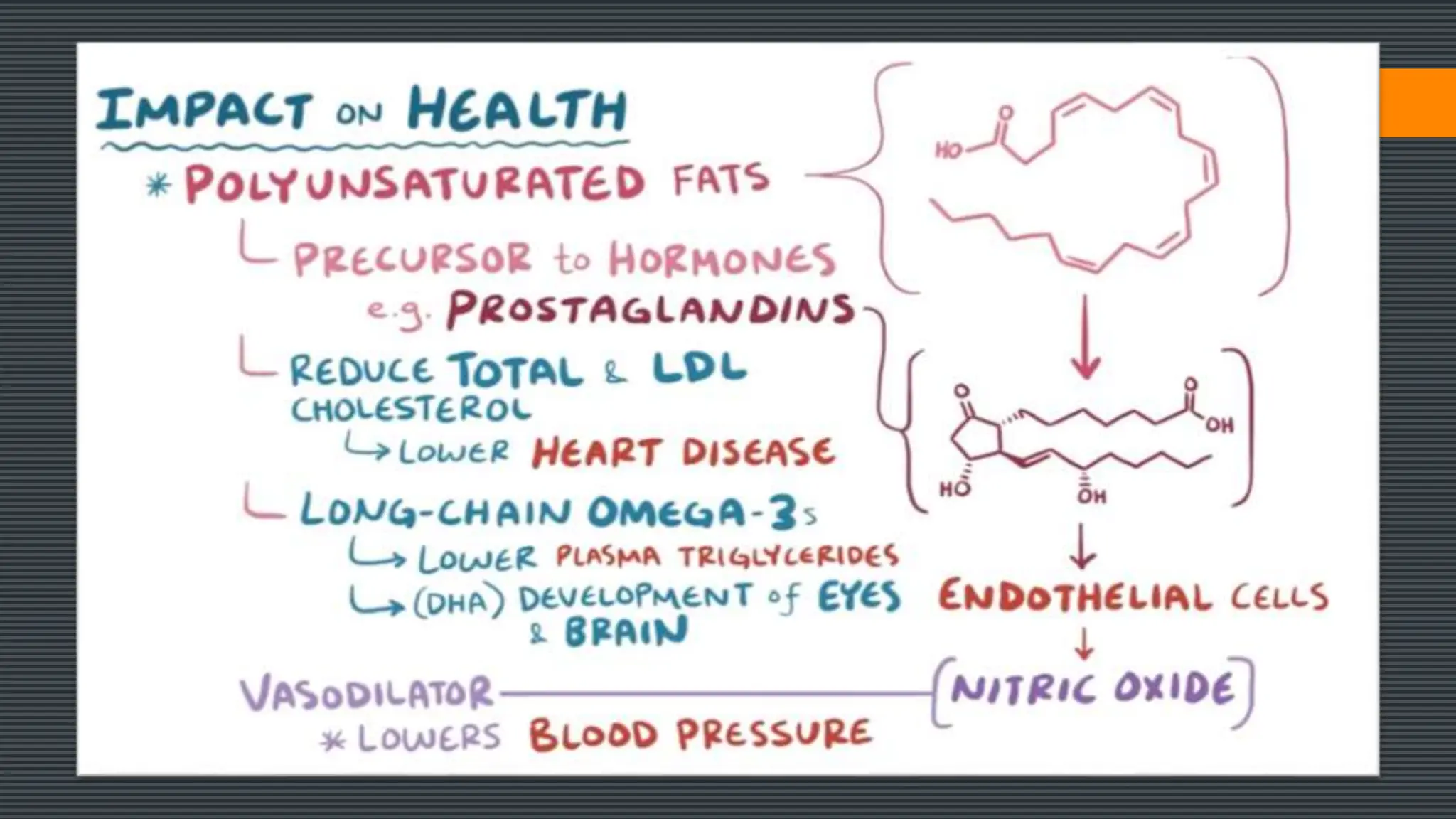
Lipids are a heterogeneous group of compounds that include fats, oils, sterols, waxes, and related compounds. They share physical properties of being relatively insoluble in water and soluble in nonpolar solvents. There are four main groups of lipids: fatty acids, glycerides, nonglyceride lipids (such as sphingolipids and steroids), and complex lipids. Lipids serve important biological roles as an energy source, for energy storage, as structural components of cell membranes, and as hormones. They are also involved in vitamin transport and absorption, protection, insulation, and other processes.