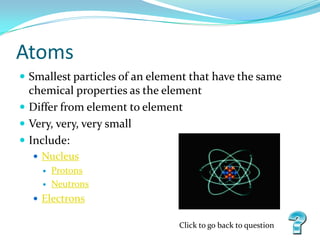
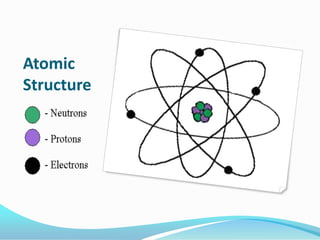
The document discusses the basics of matter, elements, atoms, and the periodic table of elements. It defines matter as anything that has mass and takes up space, and explains that matter is made up of elements, which can be solids, liquids or gases. It then describes atoms as the smallest particles that make up elements, containing a nucleus with protons and neutrons surrounded by electrons. Finally, it provides an overview of the periodic table, how it is organized into periods and families, and the types of information it contains about each element.